Navigating Payer Mandates and Staying a Step Ahead
Laure DuBois, PharmD, BCOP
Pharmacy Manager for Oncology Services
The University of Kansas Health System
Kansas City, Kansas
Nikki Ogle, PharmD, BCOP
Team Lead Reimbursement Oncology Pharmacist
The University of Kansas Health System
Kansas City, Kansas
Introduction
In the oncology landscape, payer issues can be commonplace. Some of the issues that we face as pharmacists include step edits and site of care restrictions. The goals of these restrictions are to help reduce costs and to improve patient outcomes, but they often impede patient care by slowing down the authorization process, causing confusion for financial teams, and change rapidly. To combat these challenges, our institution has implemented several processes.
Background on Step Edits
Step edits, also known as “fail first” edits, require a patient to try an alternative therapy that is usually less costly before using the therapy that was originally prescribed. In 2018, the Centers for Medicare & Medicaid Services (CMS) announced that they would use step edits for Medicare Advantage Plans in patients with cancer. 1 This was then expanded to include Medicare Part B in 2019. Once this started, many commercial payers followed suit.
When biosimilars came to the market, they presented a new angle for payers to include step edits in their contracts. Many payers have preferred biosimilars that a patient must try and fail before receiving another biosimilar or the reference product.
Key Considerations on Step Edits
There are many considerations when navigating step edits, including patient implications and authorization considerations, stocking and storing, and preventing medication errors.
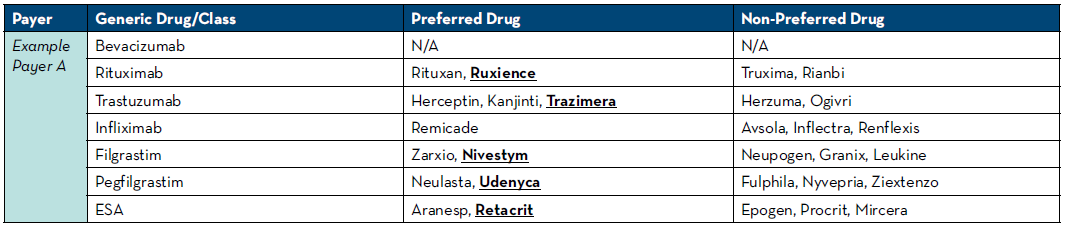
For insurance to cover the cost of an agent, your institution must obtain authorization for use prior to starting therapy. Running into a step edit at the time of authorization may lead to patient delays as this often puts the authorization process back at the beginning when requesting the new preferred agent. We have leveraged technology to assist with this and have rules written in our electronic medication record (EMR) system to look at the patient’s payers and automatically select the known mandated drug. If payers do not have a preference, we default the rule to our institutional-preferred product. To do this, we pull payer guidelines at least quarterly and keep an updated spreadsheet of preferred and non-preferred agents with the payers seen at our health system (Table 1). Another option if rules are unavailable would be to place communication orders into order sets alerting providers of which medication will be approved based on payer. Engaging industry partners is very helpful to know about changes to payer policies as they are usually knowledgeable about their medication being moved to and from preferred and non-preferred statuses.
Another step we implemented was a biosimilar auto-substitution policy. This policy allows our front-line pharmacists to change the biosimilar to the preferred product that we may only learn during the process of obtaining the authorization. We have found that the pharmacist is usually able to adjust these medications and select the right biosimilar in a timelier fashion than the physician in the clinic.
Step edits can also mandate using a generic formulation of a product instead of a brand name product. This can have downstream effects on patients if they were utilizing any form of medication assistance that is not available for generic medications. There are also times when a step edit may not be appropriate for a patient. For example, we have been asked to change pegfilgrastim to a preferred filgrastim product. In these cases, you often must go through the peer-to-peer process to get the agent you are wishing to use approved which can lead to delays in getting patients started on therapy.
Biosimilars are nightmares for look-alike sound-alike errors. To help mitigate these errors, consider only stocking your institutional-preferred biosimilar in your automated dispensing cabinets. Safety tools, like barcode medication administration, are another added layer of defense for these errors. When storing inside the pharmacy, consider placing biosimilars on different shelves and not next to the other biosimilar products. Narrowing down to one preferred agent that is accepted by payers for most patients can be helpful to decrease storing multiple biosimilars.
Background on Site of Care Restrictions
We all dread hearing the words “alternative site of care needed.” There are emerging trends in the oncology world where payers are wanting a lower site of care than a hospital outpatient department (HOD). These site of care restrictions are often targeted at supportive care medications, monoclonal antibodies, complement inhibitors, and immunotherapy.
Table 1: Preferred and Non-Preferred Agent Reference
Key Considerations on Site of Care Restrictions
We have implemented utilizing our home infusion pharmacy and/or self-injection when possible. Centers that currently do not offer home infusion services may need to start considering the pros and cons of these services. Many therapies that are not covered in HOD locations are able to be serviced by home infusion. For our cancer care partnership with home infusion, we started small and progressed to more complicated targeted agents. An easy start included medications such as IVIG, antibiotics, and growth factors. As we started to expand to targeted therapies, we made the decision to utilize our EMR system to help us with the process.
We learned quickly that oncologists like to see everything that was occurring with the patient when they transferred to home infusion. We built-out home infusion as their own treatment department with a schedule that is viewable by anyone at the institution to mirror our treatment room in the cancer center. We utilized our order sets that already existed and built-out take-home prescription orders that could be e-scribed to our home infusion pharmacy. This increased satisfaction from the oncology team as they could see where patients were in therapy and were able to visually track their cycles of therapy.
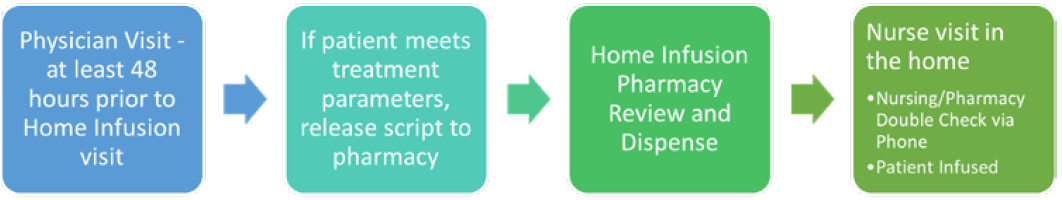
After receiving feedback from physicians and patients, we adjusted some of our home infusion workflows. To allow home infusion time to prepare for a nurse visit in the house with an accurate dose, we implemented at least a 48-hour window from physician visit to administration of the medication in the home. This allowed for dose changes and pharmacy double checks for high-risk medications. To allow for a dual verification of immunotherapy in the home we implemented a process where the home infusion nurse calls the home infusion pharmacy to verify patient, dose, vital signs, and treatment parameters were met prior to infusing the medication (Figure 1).
Home infusion services do have pitfalls. One thing to keep in mind is that most payers will only authorize one site of care, so patients are either in the infusion room or home infusion. Patients with Medicare are not covered by home infusion. Pharmacists should continue to try to persuade lawmakers to change this policy as this patient population would likely have great benefits in quality of life utilizing home infusion. Most home infusion services do have the option to see if coverage can be obtained through medical or prescription benefits. Pharmacies should review both options in order to get patients the most affordable care possible.
Another common site of care restriction is on growth factors. Often times our home infusion or specialty pharmacy can fill these and a patient can self-inject at home. For patients that are mandated to self-inject, the treatment nurse completes a teaching session with a syringe simulator on their first day of treatment. This ensures that the patient is competent to administer the medication in the home. The day after the dose is due, our home infusion pharmacy team calls to verify that the patient has administered the growth factor as a safety check.
Conclusions
Step edits and site of care restrictions are two major payer challenges in the oncology landscape. For step edits which are hard to stay on top off, implement a tracking plan to review payer policies at least twice a year if not quarterly and engage industry partners to help inform you of changes with their products. For site of care restrictions, get creative with how to keep these patients in-house and consider home infusion, self-injection, and utilizing your specialty pharmacy services.
Figure 1: Cancer Care and Home Infusion Collaboration Workflow
REFERENCES
- Modernizing Part D and Medicare Advantage To Lower Drug Prices and Reduce Out-of-Pocket Expenses. https://www.federalregister.gov/documents/2019/05/23/2019-10521/modernizing-part-d-and-medicare-advantage-to-lower-drug-prices-and-reduce-out-of-pocket-expenses. Accessed June 5, 2022.
