Tebentafusp-tebn: Cytokine Release Syndrome Management and Operational Considerations
Ian Watson, PharmD
Clinical Oncology Pharmacist
M Health Fairview University of Minnesota Medical Center
Minneapolis, MN
Paul Morales, PharmD, BCOP
Pharmacy Manager
M Health Fairview Clinics and Surgery Center
Minneapolis, MN
Uveal Melanoma Treatment Landscape
Uveal melanoma, a distinct subset of melanoma, is the most common type of primary intraocular malignancy in adults.1 The reported incidence rates of uveal melanoma are 5.74 and 7.30 cases per million in North America and Europe respectively.2 Up to 50% of patients will have metastases, and the disease has a historically poor clinical response to systemic treatment.1,3 Meta-analyses combining results across studies of chemotherapy in metastatic uveal melanoma report median overall survival (OS) ranging from 9 to 11 months.1 Meta-analyses of anti-cytotoxic T-lymphocyte associated protein 4 (CTLA-4) therapy resulted in similar or worse outcomes compared to conventional chemotherapy.1 Mixed results have been seen with other immune checkpoint inhibitor regimens in this setting with positive results limited to phase 2 trials.1 The recent approval of tebentafusp-tebn (tebentafusp) by the United States Food and Drug Administration (FDA) offers the first treatment option specifically indicated for uveal melanoma. Tebentafusp is a bispecific Human Leukocyte Antigen (HLA)-A*02:01 directed CD3 T-cell engager.4 In the general uveal melanoma population, about 45% of patients are HLA-A*02:01-positive as determined by blood test.3 The drug has unique characteristics and a side effect profile that requires careful consideration when implemented into clinical practice.
Phase 3 Trial of Tebentafusp in Metastatic Uveal Melanoma3
A multicenter, randomized, phase 3 trial compared tebentafusp with investigator’s choice of single-agent pembrolizumab, ipilimumab, or dacarbazine as first-line systemic therapy in HLA-A*02:01-positive unresectable or metastatic uveal melanoma adult patients. The phase 3 trial included 378 patients assigned in a 2:1 ratio to tebentafusp or control. Tebentafusp was dosed once weekly with initial doses of 20 micrograms (mcg) on Week 1, 30 mcg on Week 2, followed 68 mcg weekly thereafter. The administration of tebentafusp resulted in a statistically significant improvement in median OS (21.7 months vs 16.0 months; Hazard Ratio [HR] for death 0.51, 95% Confidence Interval [CI] 0.37 – 0.71) and median progression-free survival (3.3 months vs 2.9 months; HR for disease progression or death 0.73, 95% CI 0.58 – 0.94).
Cytokine release syndrome (CRS) in the tebentafusp group was common, occurring in 89% of patients. The maximum grade of CRS was Grade 1 in 12% of patients, Grade 2 in 76%, Grade 3 in 1% (2 patients), and zero patients having Grade 4 or 5. Skin-related adverse events were also common and included rash (83%), pruritus (69%), and erythema (23%). This included a 21% incidence of Grade 3 skin reactions. No Grade 4 or 5 skin reactions or cases of Stevens-Johnson syndrome or toxic epidermal necrolysis were observed. The median time to onset of skin reactions was one day and most resolved to less than Grade 1 between tebentafusp doses. The incidence of these skin-related adverse events decreased with severity and frequency with subsequent infusions. Antihistamine and topical or systemic steroids were used to treat skin reactions based on persistence and severity of symptoms. No patients discontinued therapy due to skin reactions. Increases in alanine aminotransferase or aspartate aminotransferase occurred in 65% of patients with a 3% and 4% incidence of Grade 3 or higher respectively.
Tebentafusp-associated Cytokine Release Syndrome (CRS)
CRS is defined by the American Society for Transplantation and Cellular Therapy as “a supraphysiologic response following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells."5 Further, “symptoms can be progressive, must include fever at the onset, and may include hypotension, capillary leak (hypoxia) and end organ dysfunction.”5 CRS has been previously associated with chimeric antigen receptor (CAR) T-cell therapies and the only other FDA-approved bispecific T cell engager, blinatumomab.5
Since the approval of the first CAR T-cell therapy, there have been a few different methods used for CRS grading. The first five approved CAR T-cell therapies used the Lee 2014 criteria to grade CRS in their approval studies and thus use these criteria in their respective package inserts to align CRS grade with specific management recommendations.6-10 An exception is that in two of the first approval trials for tisagenlecleucel, the Penn criteria was used, which tend to assign a higher grade of CRS compared with the Lee 2014 criteria.5,6 The approval study and package insert for blinatumomab used the CTCAE version 4 criteria for CRS grading.12 In 2019, the American Society for Transplantation and Cellular Therapy (ASTCT) published consensus grading for CRS (Table 1).5 The new consensus grading has many similarities to the CTCAE criteria with the major differences being ASTCT specifically includes fever in the definition of CRS and is more specific with the types of interventions necessary in Grades 2-4.5 The ASTCT grading is also similar to the Lee 2014 criteria, however the former separates Grade 2 and 3 hypoxia by the device used to deliver oxygen (low-flow nasal cannula [≤6 L/minute] verse high-flow devices) instead of FiO2.5 Additionally, the ASTCT grading separates Grades 2-4 by the need and number of vasopressors required instead of the dose of vasopressor needed.5 Tebentafusp, as well as the most recently approved CAR T product, ciltacabtagene autoleucel, use the 2019 ASTCT consensus grading in their approval studies and package inserts.4,11
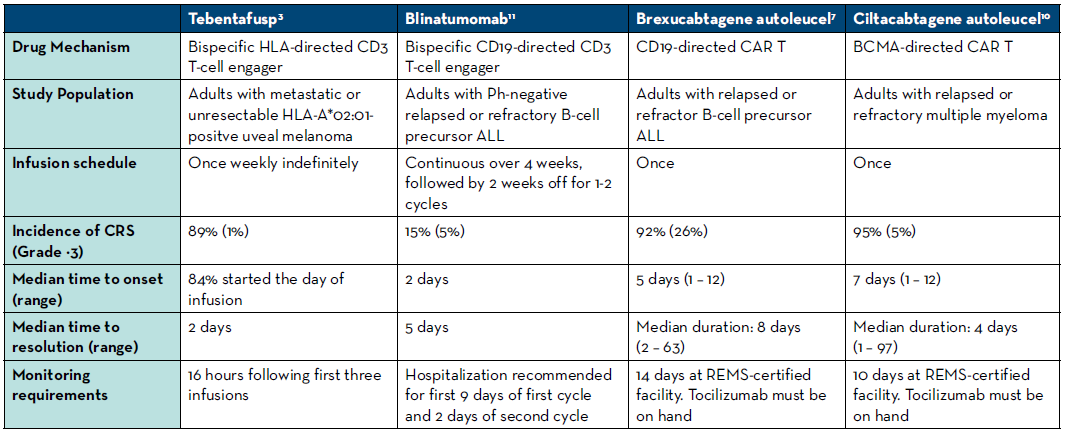
There are several important differences between the characteristics of CRS seen with tebentafusp compared to those seen with CAR T-cell therapies and blinatumomab. With tebentafusp, CRS occurred earlier (usually within a few hours after administration) and subsequently resolved earlier than with other therapies.4-12 Further characteristics of CRS are compared between a selection of these therapies in Table 2.
The treatment of CRS also varies for each therapy modality. Managing hypoxia and hypotension with oxygen supplementation and fluids or vasopressors is essential regardless of the immune therapy that caused CRS.5 Grade of CRS will need to be continually assessed based on interventions needed.5 Symptomatic treatment such as antipyretics may be beneficial for Grade 1.5 There are differences in when systemic steroids should be initiated for CRS treatment and what doses and formulations to use. Clinicians generally try to avoid corticosteroids until later in CRS associated with CAR T due to fear of causing death to the CAR T-cells and a subsequent reduction in efficacy. This risk is not a factor with the bispecific T-cell engager agents, and thus corticosteroids are often used earlier.4,12 Steroid doses and frequencies are escalated when CRS does not improve and when the grade increases.5 With tebentafusp, the manufacturer recommends administering an intravenous corticosteroid, such as methylprednisolone 2 mg/kg/ day or equivalent, beginning at Grade 3 CRS.4 The IL-6 receptor antagonist tocilizumab is an important part of CRS treatment with CAR T-cell therapies, but is not recommended for CRS of any grade associated with blinatumomab or tebentafusp.4-12 An additional consideration is the relatively short half-lives of blinatumomab and tebentafusp, which are 2.1 hours and 7.5 hours respectively. Stopping the infusion and/or delaying the next dose of these drugs can help minimize CRS duration – a strategy not available with one-time CAR T-cell infusions. Lastly, though distinct from CRS, neurotoxicity is a common and potentially severe adverse event associated with both CAR T-cell therapies and blinatumomab, but it has not been associated with tebentafusp.4-12
Operational considerations with tebentafusp
The CRS associated with tebentafusp warrants careful monitoring with appropriate tools in place to manage these events. In the Phase 3 trial, patients were monitored overnight after the first three infusions of the drug.3 The authors stated this was due to CRS events occurring in the hours after the first few doses.3 With the first three infusions of the drug the manufacturer recommends patients be monitored for 16 hours after administration, with vitals monitored at least every 4 hours.4 If a patient has not had hypotension requiring medical intervention with their most recent dose, a minimum of 30 minutes of observation after administration is required after week 4 and beyond with vitals measured twice post infusion.4 This presents a unique operational challenge as a patient will .need to receive at least their first 3 doses in a setting with nurse and provider access beyond the hours of a typical outpatient infusion center. Immediate access to emergency interventional tools including vasopressors, high flow oxygen, and mechanical ventilation with appropriately trained staff will also be necessary. The first 3 doses may need to be given in an outpatient clinic with extended hours or may require a one-night hospital admission. In this case, some institutions may be able to admit the patient after outpatient administration, under an observation status with different billing and reimbursement implications. Conversations with policy stakeholders, nursing staff, and provider groups will be important to find the optimal setting for health systems to administer tebentafusp.
Table 1. Summary of the ASTCT 2019 consensus CRS Grading5
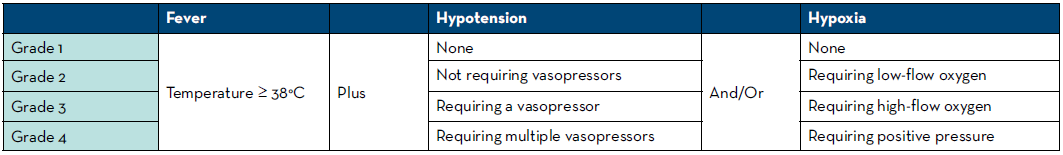
Table 2. Comparison of CRS among selected bi-specific T cell engagers and CAR T-cell therapies
Also noteworthy is the preparation instructions for tebentafusp. To prevent adsorption of the drug to the infusion bag, human albumin must be added to a bag of 0.9% Sodium Chloride before the drug is added.4 Tebentafusp is supplied in a single-dose liquid preparation and the volume to be added to the prepared infusion bag is small, between 0.1 and 0.34 milliliters, depending on the dose.4 Following preparation, a 0.2 micron in-line filter infusion set primed with 0.9% Sodium Chloride must be added.4 The final product is stable for 4 hours at room temperature, and 24 hours refrigerated.4 Like other high-cost infusion products, the cost of the drug may limit the ability of institutions to prepare the product in advance of a patient’s appointment, as avoiding waste is important.
Conclusion
Tebentafusp offers a promising treatment option in the historically challenging to treat disease state of metastatic or unresectable uveal melanoma. While it is the second bispecific T cell engager to market, its exact mechanism is novel and the nature of its side effects are unique. Practitioners can borrow from their knowledge of CRS seen with CAR T-cell therapies and blinatumomab but must be aware of important differences with tebentafusp. The monitoring requirements of tebentafusp, particularly with the first three infusions, require careful planning to ensure an institution has the resources to safely treat uveal melanoma patients. With more immune therapies in the pipeline in various disease states, continuous discussions will be necessary to determine how to best administer these products.
REFERENCES
- National Comprehensive Cancer Network Guidelines 2.2022. Melanoma: Uveal. Updated April 5, 2022. Accessed June 1, 2022. https://www.nccn.org/professionals/physician_gls/pdf/uveal.pdf.
- Naseripoor M, Azimi F, Mirshahi R, Khakpoor G, Poorhosseingholi A, Chaibakhsh S. Global Incidence and Trend of Uveal Melanoma from 1943-2015: A Meta-Analysis. Asian Pac J Cancer Prev. 2022;23(5):1791-1801. doi:10.31557/APJCP.2022.23.5.1791.
- Nathan P, Hassel JC, Rutkowski P, et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N Engl J Med. 2021;385(13):1196-1206. doi:10.1056/NEJMoa2103485.
- Kimmtrak [package insert]. Conshohocken, PA: Immunocore Commercial LLC. 2022.
- Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. doi:10.1016/j.bbmt.2018.12.758.
- Kymriah [package insert]. Conshohocken, PA: Immunocore Commercial LLC. 2022.
- Yescarta [package insert]. Santa Monica, CA: Kite Pharma, Inc. 2022.
- Tecartus [package insert]. Santa Monica, CA: Kite Pharma, Inc. 2021.
- Breyanzi [package insert]. Bothell, WA: Juno Therapeutics, Inc., a Bristol- Myers Squibb Company. 2021.
- Abecma [package insert]. Summit, NJ: Celgene Corporation, a Bristol- Myers Squibb Company. 2021.
- Carvykti [package insert]. Horsham, PA: Janssen Biotech, Inc. 2022.
- Blincyto [package insert]. Thousand Oaks, CA: Amgen Inc. 2014.
