Evaluation of Clostridioides Difficile Infection (CDI) Treatment Duration in Hematology/Oncology Patients Receiving Other Concurrent Antibiotics
Amber B. Clemmons, PharmD, BCOP, FHOPA
Clinical Professor, University of Georgia College of Pharmacy
Clinical Pharmacy Specialist
Augusta University (AU) Medical Center
Augusta, GA
Tia Stitt, PharmD
Clinical Infusion Pharmacist
Georgia Cancer Center at the AU Medical Center
Augusta, GA
Clostridioides difficile infection (CDI) is a nosocomial infection with symptoms ranging from mild to severe, including fulminant disease with toxic megacolon. Hematology/oncology patients are at higher risk for developing CDI compared to the general population (6-33% versus 1-2%).1-4 These patients are immunocompromised with an increased susceptibility to infectious processes and are frequently placed on broad-spectrum non-CDI antibiotics for prevention and treatment of bacterial infections. These agents vary in risk of developing CDI, with fluoroquinolones, cephalosporins, carbapenems, and beta-lactam/beta-lactamase inhibitors being considered high risk.5-6 Notably, these agents are frequently utilized in this patient population. Further, hematology/oncology patients are at higher risk of recurrent infections (20-40% versus 20%) which are associated with higher healthcare costs and lower quality of life.4, 7-8
Currently, the Infectious Disease Society of America (IDSA) guideline recommends treating CDI for 10-14 days.9 Additional recommended interventions include hydration and electrolyte repletion, infection control measures such as adequate handwashing, and discontinuation of non-CDI antimicrobials as soon as possible. However, the latter recommendation is challenging to implement for hematology/oncology patients given their immunocompromised state and frequent need for non-CDI antimicrobials to prevent and manage bacterial infections.
Further interventions to prevent recurrence of CDI are of much interest. Specifically, the use of CDI targeted agents has been evaluated, although only minimally in the hematology/oncology population. Prophylactic CDI treatment in patients with a history of CDI and receiving non-CDI antibiotics has been shown to be effective in a few studies.10-12 However, limited data exists regarding the extension of CDI treatment for those without history of CDI who are on concurrent non-CDI antibiotics. Due to the limited data, the IDSA guideline states that insufficient data exists to recommend extending CDI treatment beyond the recommended duration even in those patients receiving concurrent non-CDI antibiotics.9 Although minimal data is available, some providers at our institution have used prolonged or extended duration of therapy for patient on concurrent non-CDI antibiotics to prevent CDI recurrence.
To further address the impact of prolonged or extended CDI treatment in hematology/oncology patients on non-CDI antibiotics, particularly on recurrence of CDI, a joint PGY1 pharmacy residency research project was performed by Stitt and colleagues at the Phoebe Putney Memorial Hospital in Albany, Georgia along with Keats and colleagues at the AU Medical Center in Augusta, Georgia.13
This study was a multi-site, retrospective evaluation of hematology/oncology patients aged 18 years and older who were admitted to either facility between September 2013 – June 2019 with an active CDI diagnosis who received at least one dose of concurrent non-CDI antibiotics within 24 hours of CDI diagnosis or at any time during CDI treatment. Patients were classified by two different definitions for evaluation of outcomes. In the first analysis, patients were divided into cohorts by duration of CDI treatment: standard (10-14 days) versus prolonged (> 14 days). In the second planned analysis, the same patients were divided into cohorts by CDI treatment duration in relation to the end of non-CDI antibiotic treatment: non-extended (CDI treatment ended ≤ 24 hours after stopping non-CDI antibiotic) versus extended (CDI treatment ended > 24 hours after stopping non-CDI antibiotic). In both analyses, the primary outcome was incidence of CDI recurrence within 180 days of completing CDI treatment. Secondary outcomes included hospital length of stay (LOS), incidence of vancomycin-resistant enterococcus (VRE) infections at 180 days, and mortality rate at 180 days.
Table 1: Key Findings
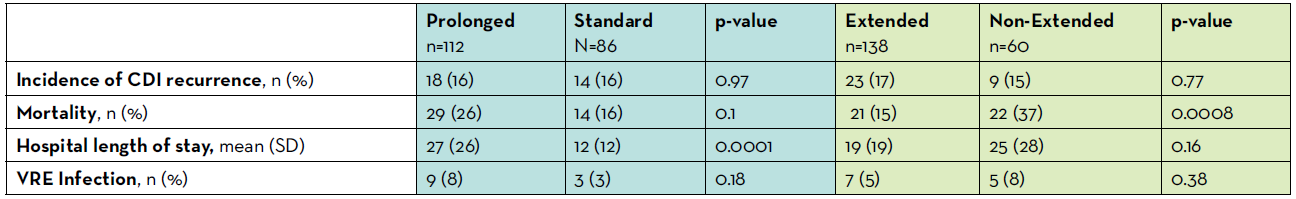
One hundred and ninety-eight patients were included in the analysis; of these, 112 were classified as prolonged duration versus 86 standard duration for the first analysis, as well as 138 classified as extended duration versus 60 non-extended duration for the second analysis. Both sets of cohorts were well balanced with respect to history of previous CDI episode, severity of CDI on diagnosis, and receipt of proton pump inhibitor. Differences in diagnosis were observed within the prolonged versus standard cohort. More patients with hematologic malignancies were included in the prolonged group and more patients with solid tumors were included in the standard group. With respect to the primary outcome, the incidence of CDI recurrence was not different between either the standard versus prolonged or between the extended versus non-extended duration cohorts (all p > 0.05). For the standard versus prolonged analysis, hospital length of stay was longer in prolonged cohort (27 vs 12 days; p<0.0001) but there was no difference in mortality (p > 0.05). For the non-extended versus extended analysis, no difference existed in hospital LOS and the mortality was lower in extended cohort (p=0.0008). Overall, a low incidence of VRE infection was documented which did not differ between standard versus prolonged cohorts (3% vs 8%; p>0.05) or between non-extended versus extended cohorts (8% vs 5%; p>0.05).
Limitations of our study include low number of patients in one of the two sites, retrospective and non-randomized nature of the study design, and inability to obtain data from outside our institutions. Ergo, under-reporting of outcomes such as CDI recurrence and death could have occurred. Further, inability to evaluate data by sub-groups, such as specific type of cancer or transplant, hinders interpretation as certain groups may be higher risk and be more likely to benefit from longer duration. Hence, further prospective and controlled trials would provide guidance to those trying to manage CDI treatment in this higher risk population. These studies should evaluate patients receiving high versus lower risk antibiotics separately, which was not able to be captured in our study due to low incidence of low-risk antibiotic use in our population. Further, future studies should evaluate specific populations at highest risk for CDI recurrence such as those with multiple risk factors or prior CDI episodes.
Providers should carefully consider the aforementioned findings before prolonging or extending the duration of CDI treatment. Given a dearth of supportive data, this practice should not be advised routinely at this time.
REFERENCES
- Kamthan AG, Bruckner HW, Hirschman SZ, et al. Clostridium difficile diarrhea induced by cancer chemotherapy. Arch Intern Med 1992; 152: 1715–1717.
- Husain A, Aptaker L, Spriggs DR, et al. Gastrointestinal toxicity and Clostridium difficile diarrhea in patients treated with paclitaxel-containing chemotherapy regimens. Gynecol Oncol 1998; 71: 104–107.
- Khan A, Raza S, Batul SA, et al. The evolution of Clostridium difficile infection in cancer patients: epidemiology, pathophysiology, and guidelines for prevention and management. Recent Pat Antiinfect Drug Discov 2012; 7: 157–170.
- Revolinski SL and Munoz-Price LS. Clostridium difficile in immunocompromised hosts: a review of epidemiology, risk factors, treatment, and prevention. Clin Infect Dis off Dis 2019; 68: 2144–2153.
- Deshpande A, Pasupuleti V, Thota P, et al. Community associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother 2013; 68: 1951–1961.
- Brown KA, Khanafer N, Daneman N, et al. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother 2013; 57: 2326–2332.
- Chung MS, Kim J, Kang JO, et al. Impact of malignancy on Clostridium difficile infection. Eur J Clin Microbiol Infect Dis 2016; 35: 1771–1776.
- Madoff SE, Urquiaga M, Alonso CD, et al. Prevention of recurrent Clostridioides difficile infection: A systematic review of randomized controlled trials. Anaerobe 2020; 61:102098.
- McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis off Dis 2018; 66: e1–e48.
- Ganetsky A, Han JH, Hughes ME, et al. Oral vancomycin prophylaxis is highly effective in preventing Clostridium difficile infection in allogeneic hematopoietic cell transplant recipients. Clin Infect Dis off Publ Infect Dis Soc Am 2019; 68: 2003–2009.
- Van Hise NW, Bryant AM, Hennessey EK, et al. Efficacy of oral vancomycin in preventing recurrent Clostridium difficile infection in patients treated with systemic antimicrobial agents. Clin Infect Dis 2016; 63: 651–653.
- Carignan A, Poulin S, Martin P, et al. Efficacy of secondary prophylaxis with vancomycin for preventing recurrent Clostridium difficile infections. Am J Gastroenterol 2016; 111: 1834–1840.
- Keats K, Stitt T, Chastain D, Jivan B, Matznick E, Waller J, Clemmons AB. Evaluating Clostridioides difficile infection (CDI) treatment duration in hematology/oncology patients receiving concurrent non-CDI antibiotics. J Oncol Pharm Pract 2021 DOI: 10.1177/1078155221998735.
