Implementation of a Pediatric-Specific Blood Factor Stewardship Program
Alexis Hamelink, PharmD
PGY2 Oncology Pharmacy Resident
Norton Children’s Hospital
Louisville, KY
Kyle Harwood, PharmD, BCPPS
Hematology/Oncology/Stem Cell Transplant Clinical Pharmacist
Norton Children’s Hospital
Louisville, KY
Joshua Elder, PharmD, BCPS, BCOP
Clinical Specialist - Pediatric Hematology/Oncology/Stem Cell Transplant
Director, PGY2 Oncology Pharmacy Residency
Norton Children’s Hospital
Louisville, KY
Introduction
Factor products are a necessary formulary inclusion within pediatric hospitals to ensure their immediate accessibility in the perioperative setting and for the treatment of trauma events in patients with bleeding disorders. The growing number of available factor products on the market, each with product-specific nuances in dosing and pharmacokinetic properties, has led to increased pharmacy department management of these products as medication therapies rather than blood products.1 Inappropriate use of factor products can also lead to notable harm events in addition to excessive expense.2
Due to the high cost, potential for error, and numerous products on the market within this class, factor stewardship has emerged as a strategy to mitigate financial toxicity while safeguarding essential resources for these patients within healthcare systems.1,3-4 However, a pediatric-specific approach to factor stewardship has yet to be described in published literature.
Implementation of a Blood Factor Stewardship Program
Factor stewardship at Norton Children’s Hospital, in Louisville, Kentucky became an integral initiative in 2013 once these products transitioned from being housed in the blood bank to the pharmacy department. Initial priority involved identifying key stakeholders to implement a factor stewardship program. Key stakeholders, which included the hematology/oncology pharmacists, pharmacy leadership, and the hematologist, met regularly to determine evidence-based strategies to optimize utilization of blood factors that were both safe and cost-effective for patient care.
The initial round of interventions in 2013 was a multifaceted approach. Transitioning blood factors to the pharmacy department allowed for prospective pharmacist review and surveillance of all blood factor-related orders. Part of this strategy involved implementing required order sets which housed all available blood factor products and included recommended dosing. A dose-rounding policy was also instituted, which allowed dose rounding up to 25% per dose to minimize waste from vials. Inventory was also shifted to a consignment system, which allowed pharmacy risk to be minimized as it related to expiration of products. Finally, dose-capping protocols were put into place with recombinant factor VIIa (NovoSeven®) which offered a maximum dose of 8 mg for hemophilia patients with inhibitors and a maximum dose of 2 mg for all other patients.
Continuous Improvement of the Blood Factor Stewardship Program
While these interventions were extremely effective and well received, in 2016 the blood factor formulary was streamlined at the institution as a means of further continuous quality improvement. The decision was made to allow for optimization of supply for the pharmacy department and for simplification of the order sets, which minimized the risk for patient safety-related events. This intervention resulted in the pharmacy department routinely stocking a single factor VIIa product, factor VIII product, a combination factor VIII/Von Willebrand factor product, and a factor IX product. Of note, appropriate reversal agents, such as prothrombin complex concentrate, were also stocked at the institution for emergent use.
Through routine monitoring of blood factor product utilization, it was noted that in 2017 and 2018 antithrombin III usage began to exponentially increase. After review of first quarter data in 2018, it was noted that antithrombin III spending was on pace to increase by 400% for the year. The factor stewardship committee, composed of the same key stakeholders listed previously, convened to propose education and use criteria, with the goal of providing more evidence-based criteria for the utility of this specific blood product. Such recommendations included a maximum dose of 1 vial (~500 units) of antithrombin III, reduction of routine monitoring of antithrombin III levels, and specific use criteria surrounding concomitant heparin doses that would necessitate the investigation/use of antithrombin III.
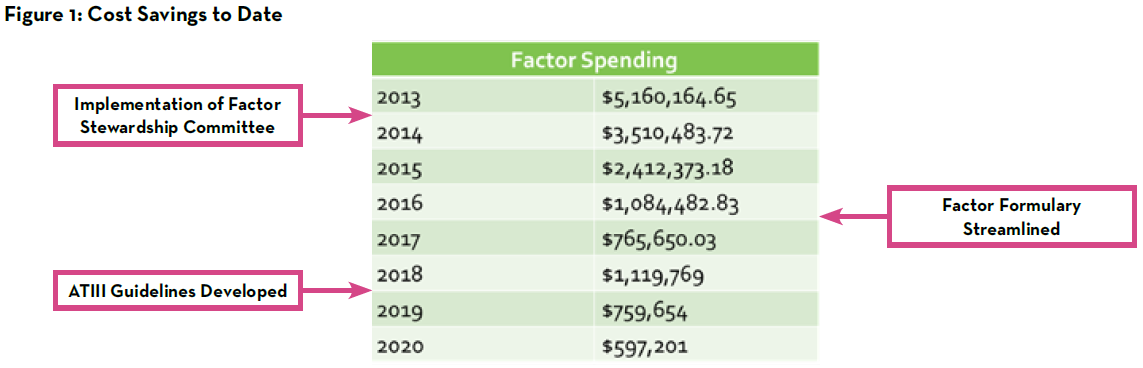
As noted in Figure 1, factor spending decreased from approximately $5.2 million annually in 2013 to approximately $600,000 annually in 2020 through the hard work of the pharmacists and other key members of the factor stewardship program. While there are certainly several interventions that can be made to optimize this work, it remains prudent to continue to closely monitor successes and further opportunities for improvement. In addition to quarterly monitoring of factor spending, patient safety reports are monitored continually to ensure no harm events arise related to blood factor utilization. In 2021, one such area that was identified at by the committee for additional optimization was the utilization of recombinant factor VIIa at our institution.
In July 2021, the factor stewardship program reduced the existing recombinant factor VIIa dose maximum of 2 mg to 1 mg for non-hemophilia patients and hemophilia patients without inhibitors. In addition to updated dose capping, staff education was provided regarding regulation of charges for factor products to optimize revenue integrity. Factor products are set to charge on dispense at Norton Children’s Hospital to ensure appropriate charges are captured for patients in the operating room, and charges must be credited to the patient if the dose was not prepared or administered.
Medication Use Evaluation Associated with the Blood Factor Stewardship Program
A medication use evaluation was performed to evaluate potential cost savings and safety events associated with the more stringent dose minimization protocol implemented by the factor stewardship committee. Doses of recombinant factor VIIa that were administered between January 1 and December 31, 2021 were included in the study. Patients with hemophilia with documented inhibitors were excluded from the evaluation, as the dose cap for these patients remained at 8 mg. A total of 46 patients with 103 recombinant factor VIIa product administrations were evaluated over the study period, with 29 of the 103 doses being administered after the dose minimization protocol was updated.
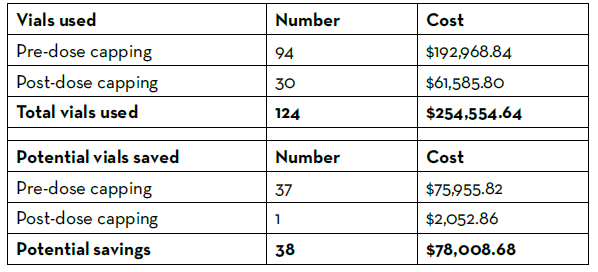
Prior to the dose minimization protocol update, 9% of doses exceeded 2 mg, and 47% of doses were between 1 mg and 2 mg. After the dose minimization protocol update, 3% of doses exceeded 1 mg. The number of recombinant factor VIIa vials used over the course of the year were tallied and recorded (Table 1). There were 124 vials used over the evaluation time period, costing the pharmacy department $254,555 using the estimated GPO cost of $2,052.86 per 1 mg vial. Thirty-eight vials could have been saved from use had the doses followed the stricter dosing protocol, leading to an estimated 1-year potential cost savings of $151,912 annually. No life-threatening safety events occurred in patients after the dose minimization protocol was updated. The medication use evaluation concluded that a maximum recombinant factor VIIa dose of 1 mg in non-hemophilia patients and hemophilia patients without inhibitors appeared to be safe and effective in the pediatric population and resulted in significant cost savings with improved revenue integrity.
Summary
Factor stewardship remains an integral effort across healthcare systems to ensuring appropriate use of these high-priced products. Although stewardship strategies have proven effective at other institutions, published guidance for a pediatric-specific program is not available.1-4 The nine years of experience at Norton Children’s Hospital has yielded an overall annual cost savings of approximately 90% since its inception from the approximately $5.2 million annual initial spending in 2013 to approximately $600,000 annual spending in 2020.
Key interventions contributing to the success of our program include dose minimization, vial size rounding, formulary streamlining, and consideration of a consignment-based ordering system.
Table 1: Recombinant Factor VIIa Vials Used and Potential Savings
Future Directions
Future directions for our program include expansion of stewardship efforts into the outpatient setting, which is challenged by the need for patient-specific factor product administration for pharmacokinetic data. The extension of stewardship efforts is aided by the development of newer subcutaneously administered outpatient therapies, such as emicizumab, that reduce the need for regular intravenous factor infusions in certain populations, including hemophilia patients with inhibitors. With further advanced therapies on the horizon and with the continually changing landscape of healthcare, continual quality improvement is necessary to achieve the best possible outcomes for our patients while promoting stewardship of resources.
REFERENCES
- Dane K, Streiff M, Lindsley J, et al. The Development and Impact of Hemostatic Stewardship Programs. Hematol Oncol Clin N Am (2019) 33:887–901.
- Trueg A, Lowe C, Kiel P, et al. Clinical Outcomes of a Pharmacy-Led Blood Factor Stewardship Program (2017) 24:643-647.
- Waheed A, Fongemie J, Gopal S, et al. Implementation and Impact of a Multidisciplinary Coagulation Factor Stewardship Program at an Academic Medical Center. Journal of Thrombosis and Thrombolysis (2020) 50:715–717.
- Amerine L, Chen S, Daniels R, et al. Impact of an Innovative Blood Factor Stewardship Program on Drug Expense and Patient Care. Am J Health- Syst Pharm (2015) 72:1579-84.
