HOPA Publications Committee
Christan M. Thomas, PharmD, BCOP, Editor
Lisa Cordes, PharmD, BCOP, BCACP, Associate Editor
Renee McAlister, PharmD BCOP, Associate Editor
Lydia Benitez, PharmD, BCOP
Alexandra Della Pia, PharmD, MBA, BCOP
Jeff Engle, PharmD, MS
Karen M. Fancher, PharmD, BCOP
Chung-Shien Lee, PharmD, BCOP, BCPS
Robert Steven Mancini, PharmD, BCOP, FHOPA
Bernard L. Marini, PharmD, BCOP
Alan L. Myers, PharmD, PhD
Gregory T. Sneed, PharmD
Diana Tamer, PharmD, BCOP
Kristin Held Wheatley, PharmD, BCOP
HOPA News Staff
David DeRemer, PharmD, BCOP, FCCP, FHOPA, Board Liaison
Michelle Sieg, Communications Director
Joan Dadian, Marketing Manager
View PDF of HOPA News, Vol. 19, no. 2
Board Update: Four Focus Areas for 2022-2023
Heidi D. Finnes, PharmD, BCOP, FHOPA
HOPA President (2022–2023)
Senior Manager, Pharmacy Cancer Research
Director, Pharmacy Shared Resources, Mayo Clinic Cancer Center
Assistant Professor of Pharmacy, Mayo Clinic Alix School of Medicine
Rochester, MN
During a time of unprecedented professional burnout, HOPA leaders, members, and staff continue to work together to fulfill the mission of supporting the hematology/oncology pharmacy profession. More than 1,400 of us gathered in Boston at the end of March for Annual Conference 2022 (AC22), our first in-person learning event since COVID-19 began. Another 200 professionals participated in AC22 Encore in May. That sets a new record for annual conference attendance in our nearly 20-year history.
You Matter
I want to thank Larry Buie, Immediate Past-President, and the 2021-2022 HOPA Board of Directors for their continued leadership. I also want to thank David DeRemer, Past-President, and Patrick Medina, Treasurer, for their years of service on the HOPA Board of Directors.
Mostly, I want to thank all of you, our HOPA members, for continuing to persevere through a public health crisis and related loss and exhaustion. Your work has inspired four focus areas for the coming year.
Build on Novel COVID-19 Practices
“Pivot” is a word often used to describe what we as pharmacists and technicians had to do at the onset of COVID-19. We all pivoted to telemedicine and video visits, and many of us transitioned patients to oral treatments or extended cycle dosing of traditional chemotherapy. For some of us, the necessity of going remote has led to monitoring patient laboratory values and vitals with wearable technology.
It is important that we all continue to expand these practices – especially those that make cancer care more accessible and efficient. Can we offer telehealth for follow-ups that don’t require physical exams? Could video visits help patients on oral cancer therapies prevent toxicity? Can electronic consultations and medication reconciliation further the use of precision oncology and pharmacogenetics?
I encourage you to share your innovations in care and publish your quality and research projects! Your examples of patient advocacy throughout a global pandemic help elevate the role of the hematology oncology pharmacist in the long-run.
Expand Diversity, Equity, and Inclusion (DEI) Initiatives
When HOPA’s official DEI Statement was published late last year, it was just the beginning of our formalized commitment to patients and each other. Our DEI focus is demonstrated by how we advocate for equitable health policies and more inclusive research studies. It is seen in how we diversify our educational products and resources to reflect the needs of all members.
Thanks to the hard work of the DEI Task Force, the tenets of DEI are embedded in HOPA policies and procedures, committee and leadership requirements, and strategic planning for 2023 and beyond. The DEI Task Force, which was a time-limited initiate, is now a full committee with new members as of June 1 of this year.
Improve the Health of HOPA and our Members
The health of an association can be measured in a couple of ways – growth and stability of the organization itself, and the health, wellness, and productivity of our members. Some initiatives allow us to improve the health of both the organization and individuals.
This year, a Wellness Task Force (to combat burnout) and the HOPAmbassadors Task Force, which makes members into spokespeople, have been added to the list of volunteer engagement opportunities. Members also can still volunteer for roles in Advocacy, Student Engagement, and the Patient Advisory Panel.
Recognize and Manage Burnout
While we have all felt the fatigue of COVID-19, as hematology/oncology pharmacists, we were feeling burned out from patient care activities long before the pandemic began. Research, including from HOPA member Allison Golbach from the University of Kansas, has shown us the extent of burnout’s prevalence and impact.
Now, HOPA is poised to take the next step, which is to combat burnout with health and wellness activities to support ourselves and one another. The new Wellness Task Force will glean and share insights and recommendations from wellness consultants, including AC22 Keynote speaker J. Bryan Sexton, PhD, from the Duke Center for Healthcare Safety & Quality.
Perhaps most notably, the Wellness Task Force is charged with developing tools and resources to recognize and manage burnout.
I look forward to a productive year of leading – and learning from – each of you. Thank you for all you do to help ensure all individuals affected by cancer have a hematology/oncology pharmacist as an integral member of their care team.
Feature: The Blood Stops Here: Management of Pediatric Hemophilia in 2022
Alexis Kuhn, PharmD, BCOP
Pediatric Oncology Pharmacist- Ambulatory Service,
Assistant Professor of Pharmacy
Mayo Clinic College of Medicine, Mayo Clinic
Rochester, MN
The therapeutic landscape in the field of hematology/oncology pharmacy has drastically evolved over the past decade, with mainstream emergence of precision medicine and immunotherapies changing the paradigm of how we treat certain cancers.1 So too has the paradigm shifted in the management of persons with hemophilia; where once we could only offer short-acting clotting factor concentrates (“factors”), we can now offer non-factor therapeutics, and extended half-life factors, and we stand on the cusp of gene therapy.2
Babies with hemophilia born today have therapeutic options available to them that their uncles and grandfathers never even dreamed about. Herein we will review the management of hemophilia in pediatric patients and highlight several promising avenues that the next decade could bring.
Hemophilia 101
Congenital hemophilia is an X-linked bleeding disorder arising from genetic mutations that result in inadequate production of clotting factor.3 When the F8 gene, which is responsible for factor VIII (FVIII) production, is implicated, the clinical syndrome is termed hemophilia A; when F9, responsible for factor IX (FIX) production, is implicated, hemophilia B is the result.4 An estimated 1.25 million individuals worldwide are expected to have hemophilia, with 80-85% of those having hemophilia A.5 Given that the F8 and F9 genes are located on the X chromosome, hemophilia predominantly affects males who inherit the gene from their carrier mothers.
Both hemophilia A and B are classified by severity, which is determined by baseline factor activity level: a level of <1% indicates severe disease, 1-5% moderate disease, and 6-40% mild disease.3 Phenotypically, patients with severe disease experience spontaneous bleeds into joints and muscles, with significant morbidity and possible mortality. Patients with moderate disease tend to have fewer spontaneous bleeds but still have excessive bleeding with minor trauma, and patients with mild disease generally don’t experience spontaneous bleeds but do experience excessive bleeding from major trauma.3
Historically, treatment of hemophilia has centered around replacing the missing factor with intravenous clotting factor concentrates. The 1970s and 1980s saw the dawn of human plasma-derived factor concentrates, and so too came the emergence of blood-borne viral infections.2,4 The 1990s brought the advent of the recombinant factor concentrates, which abrogated the risk of viral transmission.4 Regardless of source, factor concentrates are administered either routinely to prevent spontaneous bleeds (‘prophylaxis’), or on-demand in response to an active bleed (‘episodic’).
For severe disease, prophylaxis has been the mainstay of treatment.6 Inhibitor development, which refers to the emergence of neutralizing antibodies against exogenous factor, is one of the most significant complications of clotting factor concentrates. Inhibitors to FVIII or FIX represent a dangerous and costly development that gravely complicates hemophilia management.7
Management of the Pediatric Patient
In its most recent 2020 guideline update, the World Federation of Hemophilia (WFH) maintains the importance of early initiation of prophylaxis for children with severe hemophilia A or B.6 Ideally, prophylaxis is initiated before the child’s 3rd birthday and prior to the development of any joint disease; early initiation of factor prophylaxis confers better long-term joint outcomes and significantly reduces the risk for intracranial hemorrhage.6,8-10 Choice of prophylaxis regimen should be tailored to the child’s and family’s needs and can be broken in two broad categories: factor and non-factor.
Factor Prophylaxis (Children without Inhibitors)
Factor prophylaxis has been the mainstay of therapy for decades, with well-known limitations. FVIII and FIX concentrates require intravenous access and frequent administration (typically 3-4 times per week for standard half-life FVIII concentrates and 2-3 times per week for standard half-life FIX concentrates), often with caregivers as the ones establishing intravenous access in their children.6 Such regimens can be unwieldy in a young child, and oftentimes central venous access devices (CVADs) are implanted to aid factor administration in the home. CVADs can be a nidus for infection and/or thromboses and are only meant to be used for the shortest period possible.
Prophylactic factor regimens can and should be personalized to the child when able. Hematology/oncology pharmacists can assist with performing pharmacokinetic studies of the child’s factor, either manually and/or ideally with the assistance of a Bayesian modeling program, such as WAPPS-Hemo.11-13
The emergence of extended half-life (EHL) factor products has been an exciting development in recent years, particularly for children with hemophilia B.14 The half-life of a clotting factor can be prolonged by either conjugating the factor with polyethylene glycol (PEG) or fusing the factor to either albumin or the Fc component of IgG1. The degree to which the half-life is extended differs between the factors: for currently licensed EHL FIX products, the half-life is extended ~4-5x from that of a standard half-life FIX; for currently licensed EHL FVIII products, the half-life is only extended ~1.5-fold.14 For children with hemophilia B, this translates to the attractive possibility of once-weekly FIX prophylaxis.15 For children with hemophilia A, despite the only modest half-life extension, this has the potential to translate to one less factor infusion per week— which can be meaningful for the child and caregiver alike.16
Non-Factor Prophylaxis (Children with Hemophilia A with or without Inhibitors)
In 2017, emicizumab-kxwh ushered in a new era in hemophilia management, becoming the first non-factor prophylactic therapy approved by the United States Food and Drug Administration (FDA).17 Emicizumab is a bispecific monoclonal antibody that mimics FVIII function but shares no homology to the native protein, making it a viable therapeutic in the presence of inhibitors.17 Its initial approval was in hemophilia A with inhibitors, followed shortly thereafter by its approval in hemophilia A without inhibitors. The pivotal HAVEN trial series generated efficacy and safety data to support its licensing in children and adults alike, with or without inhibitors, and at an array of dosing regimens.18-21
Since the original HAVEN 2 publication in children with inhibitors, a growing number of publications have described the successful use of emicizumab in pediatric patients ranging from infancy to adolescence.19, 22-26 Unlike intravenous factor concentrates, emicizumab is administered subcutaneously. The subcutaneous route of administration is especially attractive in very young children for whom a CVAD would have otherwise been needed and makes prophylaxis reasonably attainable in this vulnerable population.24
Emicizumab dosing is weight-based, and after a standard 4-week loading period, maintenance doses can be administered every 1, 2, or 4 weeks at a dose of 1.5 mg/kg, 3 mg/kg, or 6 mg/kg, respectively.27 As the child grows, clinicians will need to continually adjust doses to fit the child; a variety of vial sizes exist to achieve a target dose. It is important to note that unlike factor VIII concentrates, with which caregivers are generally advised to use a whole vial, partial vials of unused emicizumab should be discarded.
Future Directions
The next decade promises to build upon the era that emicizumab has brought forth, as several novel therapeutics are currently undergoing late stage development. Concizumab is a monoclonal antibody directed against tissue factor pathway inhibitor (TFPI).28 TFPI inhibition yields increased thrombin generation, and as part of the extrinsic pathway of the coagulation cascade, is a viable therapeutic target for both hemophilia A and B.29 Concizumab is administered as a daily subcutaneous injection and has achieved FDA Breakthrough Therapy designation for hemophilia B with inhibitors.28
An alternate approach to increase thrombin generation is to decrease antithrombin expression. Fitusiran is a small interfering RNA that targets antithrombin mRNA in hepatocytes.30 Like concizumab, fitusiran demonstrates activity in both hemophilia A and B and is administered subcutaneously. The ATLAS-PEDS trial (NCT03974113) is actively recruiting boys 1-12 years of age with hemophilia A or B to receive fitusiran prophylaxis.
Gene therapy is perhaps the most highly anticipated development in hemophilia, with several candidate products having completed enrollment in their respective phase 3 trials.31-33 Hemophilia gene therapy involves the administration of a modified transgene (either F8 or F9, often paired with a liver-specific promoter) enveloped in a viral vector.34 Most candidate products to date rely on an adeno-associated virus (AAV) vector platform. This is advantageous as it circumvents the insertional mutagenesis possible with lentiviral vectors, but is potentially problematic in children as their episomal nature may predispose to diluting out as the child (and his liver) grows over time.31 Children have been excluded from hemophilia gene therapy trials to date.
Conclusions
In summary, with novel therapeutics and gene therapies on the horizon, coupled with the present-day reality of EHL factors and emicizumab, babies born with hemophilia today do not have to accept the fate of generations before them. As the paradigm continues to shift and novel therapies continue to emerge, hematology/oncology pharmacists must stay abreast of current literature to provide attentive and compassionate care to these patients.
REFERENCES
- Islami F, Seigel RL, Jemal A. The changing landscape of cancer in the USA—opportunities for advancing prevention and treatment. Nature Rev Clin Oncol 2020; 17:631-49.
- Mancuso ME, Mahlangu JN, Pipe SW. The changing treatment landscape in haemophilia: from standard half-life clotting factor concentrates to gene editing. Lancet 2021; 397(10274):630-640.
- Srivastava A, Brewer AK, Mauser-Bunschoten EP et al. Guidelines for the management of hemophilia. Haemophilia 2013; 19(1):e1-47.
- Mannucci PM, Tuddenham EG. The hemophilias—from royal genes to gene therapy. N Eng J Med 2001; 344(23):1773-9.
- Iorio A, Stonebraker JS, Chambost H et al. Establishing the prevalence and prevalence at birth of hemophilia in males: a meta-analytic approach using national registries. Ann Intern Med 2019; 171(8):540-546.
- Srivastava A, Santagostino E, Dougall A et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia 2020; 26 Suppl 6:1- 158.
- Ljung R, Auerswald G, Benson G et al. Inhibitors in haemophilia A and B: management of bleeds, inhibitor eradication and strategies for difficult-to-treat patients. Eur J Haematol 2019; 102(2):111-122.
- Manco-Johnson MJ, Abshire TC, Shapiro AD et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Eng J Med 2007; 357(6):535-44.
- Andersson NG, Auerswald G, Barnes C et al. Intracranial haemorrhage in children and adolescents with severe haemophilia A or B – the impact of prophylactic treatment. Br J Haematol 2017; 179(2):298-307.
- Fischer K, Collins PW, Ozelo MC et al. When and how to start prophylaxis in boys with severe hemophilia without inhibitors: communication from the SSC of the ISTH. J Thromb Haemost 2016; 14(5):1105-9.
- Shapiro AD, Korth-Bradley J, Poon MC. Use of pharmacokinetics in the coagulation factor treatment of patients with haemophilia. Haemophilia 2005; 11:571-582.
- Dargaud Y, Delavenne X, Hart DP et al. Individualized PK-based prophylaxis in severe haemophilia. Haemophilia 2018; 24 Suppl 2:3-17.
- Hajducek D, Chelle P, Hermans C et al. Development and evaluation of the population pharmacokinetic models for FVIII and FIX concentrates of the WAPPS-Hemo project. Haemophilia 2020; 26(3):384-400.
- Mannucci P. Hemophilia therapy: the future has begun. Haematologica 2020; 105(3):545-553.
- Kenet G, Chambost H, Male C et al. Long-acting recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in children. Thromb Haemost 2016; 116:659-668.
- Mullins ES, Stasyshyn O, Alvarez-Roman MT et al. Extended half-life pegylated, full-length recombinant factor VIII for prophylaxis in children with severe haemophilia A. Haemophilia 2017; 23:238-246.
- Scott LJ, Kim ES. Emicizumab-kxwh: first global approval. Drugs 2018; 78(2):269-274.
- Oldenburg J, Mahlangu JN, Kim B et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med 2017; 377:809-818.
- Young G, Liesner R, Chang T et al. A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood 2019; 134(24):2127-2138.
- Mahlangu J, Oldenburg J, Paz-Priel I et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med 2018; 379:811-822.
- Pipe SW, Shima M, Lehle M et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open-label, non-randomised phase 3 study. Lancet Haematol 2019; 6(6):e295-e305.
- Shima M, Nogami K, Nagami S, et al. A multicentre, open-label study of emicizumab given every 2 or 4 weeks in children with severe haemophilia A without inhibitors. Haemophilia 2019;25(6):979-987.
- Cohen CT, Diaz R. Emicizumab in pediatric hemophilia: bleeding and surgical outcomes from a single-center retrospective study. Pediatr Blood Cancer 2021; 68(11)e29325.
- Mason JA, Young G. Emicizumab prophylaxis in infants with severe haemophilia A without inhibitors: illustrative real-world cases to support shared decision-making. Haemophilia 2021; 27(5):724-29.
- Garcia J, Zia A. Real-world case series and summary of current literature of infants and toddlers with severe hemophilia A with inhibitor on prophylaxis with emicizumab. Pediatr Blood Cancer 2021; 68(5):e28942.
- Batsuli G, Greene A, Meeks SL et al. Emicizumab in tolerized patients with hemophilia A with inhibitors: a single-institution pediatric cohort assessing inhibitor status. Res Pract Thromb Haemost 2021; 5(2):342-348.
- Hemlibra [package insert]. South San Francisco, CA: Genentech, Inc.; 2021.
- Shapiro AD. Concizumab: a novel anti-TFPI therapeutic for hemophilia. Blood Adv 2021; 5(1):279.
- Shapiro AD, Angchaisuksiri P, Astermark J et al. Long-term efficacy and safety of subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors. Blood Adv 2022; bloodadvances.2021006403. [online ahead of print]
- Manchin N, Ragni MV. An investigational RNAi therapeutic targeting antithrombin for the treatment of hemophilia A and B. J Blood Med 2018; 9:135-140.
- Batty P, Lillicrap D. Hemophilia gene therapy: approaching the first licensed product. HemaSphere 2021; 5(3):e540.\
- Pipe SW, Leebeek FW, Recht M et al. 52 week efficacy and safety of etranacogene dezaparvovec in adults with severe or moderate-severe hemophilia B: data from the phase 3 HOPE-B gene therapy trial [abstract]. Res Pract Thromb Haemost 2021; 5(Suppl 2).
- Ozelo MC, Mahlangu J, Pasi KJ et al. Valoctocogene roxaparvovec gene therapy for hemophilia A. N Eng J Med 2022; 386:1013-1025.
- Pierce GF. Gene therapy for hemophilia: anticipating the unexpected. Blood Adv 2020; 4(15):3788.
Setting Boundaries after Babies
Kristin Held Wheatley, PharmD, BCOP
Clinical Pharmacy Specialist, Pediatric Oncology and Infectious Diseases
Program Director, PGY1 Pharmacy Residency
Lehigh Valley Health Network
Allentown, PA
Chung-Shien Lee, PharmD, BCOP, BCPS
Associate Professor
St John’s University College of Pharmacy
Queens, NY
“I’ve learned that you can’t have everything and do everything at the same time.”
— Oprah Winfrey
Many of us are overextended and this feeling can be magnified when starting a family. Here, two clinicians share their perspectives on navigating a new normal. And, you may find their advice about balancing—rather than juggling—your home and work lives useful, even if you aren’t preparing for a baby.
Kristin Held Wheatley, PharmD, BCOP
I used to define myself by my job and volunteered for every opportunity that came my way. I was one of the first to arrive, the last to leave, and frequently took my laptop home to work some more. That was until the unexpected happened—I was expecting.
At the first nurse visit they estimated my delivery date. My mind immediately calculated three months from that date, and I was relieved. I would be back from leave before my current Post-Graduate Year 1 (PGY1) residents graduated and would have time to prepare to onboard the incoming residents. And so began my preparation for the time I anticipated being away.
My best advice is to be realistic with your priorities, both professional and personal. I have a passion for giving back through leadership roles within professional organizations. However, one role no longer aligned with my professional goals or interests. And while I struggled penning my resignation, it was freeing. Shortly after, I was offered a leadership opportunity with a different organization that excited me and would open doors for me in the future. But I was nervous that my upcoming leave would place unnecessary hardship on the committee chair. To my surprise, the current leadership shared in my excitement and were happy to carry the weight while I was gone.
I had to follow a similar mentality when preparing for my maternity leave and the list seemed endless—what needed to get done, what would need to be covered, and who was going to cover it? I started asking for timelines when they weren’t provided. Some were clarified, others were hastened, and some were flat out refused! Yes, I HAD to learn to say No. I provided an alternate contact or quickly sent my thoughts, but it was complete shift in mindset for me. I’d like to say I was able to accomplish everything on my to do list before I went out on leave but that was impossible, and everything was left standing despite my departure!
I always knew I’d return to work full-time. I love what I do too much to step away. My greatest struggle has been getting comfortable with less progress. I’m responsible for drop-off and pick-up and I’ve had to transition leaving my desk no later than 5 p.m. for a 5:30 p.m. pick-up. There are times when I must take things home, but my laptop is left at my desk more often than it travels back and forth. I’ve decided to prioritize my family rather than burning the midnight oil on deadlines that will be replaced by new ones the next day. There will continue to be an ebb and flow between work and home responsibilities, but I’m committing to being present at home and keeping work at work as much as I can.
It is possible to be both a good clinician and an involved parent—though it has required adjustment!
Chung-Shien Lee, PharmD, BCOP, BCPS
Like Dr. Wheatley, I too was often overly ambitious with my work and career. It was the norm to work extended hours and take work home with me. I looked forward to volunteering in numerous committees and organizational activities. This all changed after the birth of my first child.
For most of my professional career, I’d often plan and juggle things around work, but for the first time in my life this was no longer the case. I learned quickly that I would now have to juggle things around my family.
Setting boundaries was a new skill that I had to fine tune. For many of us who are motivated and ambitious, taking a pause or slowing down in our careers is a big adjustment. In the initial months approaching the due date, I anticipated taking a brief pause and being able to resume business as usual. However, this was not the case; and the pause ended up being longer than initially planned for many reasons. Many projects and manuscripts I was working on needed to be delayed. I could no longer catch up on things at home during off hours like I usually did.
Having recently welcomed my second child, my approach was different the second time around.
My advice to anyone who is adjusting to both a career and family life is to live in the moment. When you are away from work and spending time with your family, be there mentally and physically. This is a challenge for many of us, including myself still, but the attention you provide to your kids goes a long way.
When expecting, I’d encourage new parents to hold off on starting anything new or overextending yourself. This is a good time to prepare for the new addition and the adjustments to come. Many workplaces are now offering paid family leave, which I highly recommend taking. Luckily for me, the second time around I could take this leave, which has helped in some ways draw the boundaries when not physically at work.
“Don’t get so busy making a living that you forget to make a life.”
— Dolly Parton
Implementation of a Pediatric-Specific Blood Factor Stewardship Program
Alexis Hamelink, PharmD
PGY2 Oncology Pharmacy Resident
Norton Children’s Hospital
Louisville, KY
Kyle Harwood, PharmD, BCPPS
Hematology/Oncology/Stem Cell Transplant Clinical Pharmacist
Norton Children’s Hospital
Louisville, KY
Joshua Elder, PharmD, BCPS, BCOP
Clinical Specialist - Pediatric Hematology/Oncology/Stem Cell Transplant
Director, PGY2 Oncology Pharmacy Residency
Norton Children’s Hospital
Louisville, KY
Introduction
Factor products are a necessary formulary inclusion within pediatric hospitals to ensure their immediate accessibility in the perioperative setting and for the treatment of trauma events in patients with bleeding disorders. The growing number of available factor products on the market, each with product-specific nuances in dosing and pharmacokinetic properties, has led to increased pharmacy department management of these products as medication therapies rather than blood products.1 Inappropriate use of factor products can also lead to notable harm events in addition to excessive expense.2
Due to the high cost, potential for error, and numerous products on the market within this class, factor stewardship has emerged as a strategy to mitigate financial toxicity while safeguarding essential resources for these patients within healthcare systems.1,3-4 However, a pediatric-specific approach to factor stewardship has yet to be described in published literature.
Implementation of a Blood Factor Stewardship Program
Factor stewardship at Norton Children’s Hospital, in Louisville, Kentucky became an integral initiative in 2013 once these products transitioned from being housed in the blood bank to the pharmacy department. Initial priority involved identifying key stakeholders to implement a factor stewardship program. Key stakeholders, which included the hematology/oncology pharmacists, pharmacy leadership, and the hematologist, met regularly to determine evidence-based strategies to optimize utilization of blood factors that were both safe and cost-effective for patient care.
The initial round of interventions in 2013 was a multifaceted approach. Transitioning blood factors to the pharmacy department allowed for prospective pharmacist review and surveillance of all blood factor-related orders. Part of this strategy involved implementing required order sets which housed all available blood factor products and included recommended dosing. A dose-rounding policy was also instituted, which allowed dose rounding up to 25% per dose to minimize waste from vials. Inventory was also shifted to a consignment system, which allowed pharmacy risk to be minimized as it related to expiration of products. Finally, dose-capping protocols were put into place with recombinant factor VIIa (NovoSeven®) which offered a maximum dose of 8 mg for hemophilia patients with inhibitors and a maximum dose of 2 mg for all other patients.
Continuous Improvement of the Blood Factor Stewardship Program
While these interventions were extremely effective and well received, in 2016 the blood factor formulary was streamlined at the institution as a means of further continuous quality improvement. The decision was made to allow for optimization of supply for the pharmacy department and for simplification of the order sets, which minimized the risk for patient safety-related events. This intervention resulted in the pharmacy department routinely stocking a single factor VIIa product, factor VIII product, a combination factor VIII/Von Willebrand factor product, and a factor IX product. Of note, appropriate reversal agents, such as prothrombin complex concentrate, were also stocked at the institution for emergent use.
Through routine monitoring of blood factor product utilization, it was noted that in 2017 and 2018 antithrombin III usage began to exponentially increase. After review of first quarter data in 2018, it was noted that antithrombin III spending was on pace to increase by 400% for the year. The factor stewardship committee, composed of the same key stakeholders listed previously, convened to propose education and use criteria, with the goal of providing more evidence-based criteria for the utility of this specific blood product. Such recommendations included a maximum dose of 1 vial (~500 units) of antithrombin III, reduction of routine monitoring of antithrombin III levels, and specific use criteria surrounding concomitant heparin doses that would necessitate the investigation/use of antithrombin III.
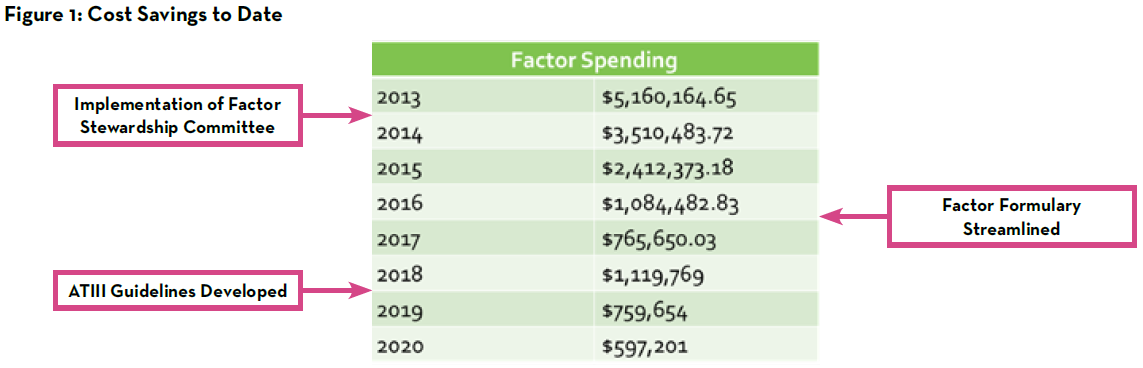
As noted in Figure 1, factor spending decreased from approximately $5.2 million annually in 2013 to approximately $600,000 annually in 2020 through the hard work of the pharmacists and other key members of the factor stewardship program. While there are certainly several interventions that can be made to optimize this work, it remains prudent to continue to closely monitor successes and further opportunities for improvement. In addition to quarterly monitoring of factor spending, patient safety reports are monitored continually to ensure no harm events arise related to blood factor utilization. In 2021, one such area that was identified at by the committee for additional optimization was the utilization of recombinant factor VIIa at our institution.
In July 2021, the factor stewardship program reduced the existing recombinant factor VIIa dose maximum of 2 mg to 1 mg for non-hemophilia patients and hemophilia patients without inhibitors. In addition to updated dose capping, staff education was provided regarding regulation of charges for factor products to optimize revenue integrity. Factor products are set to charge on dispense at Norton Children’s Hospital to ensure appropriate charges are captured for patients in the operating room, and charges must be credited to the patient if the dose was not prepared or administered.
Medication Use Evaluation Associated with the Blood Factor Stewardship Program
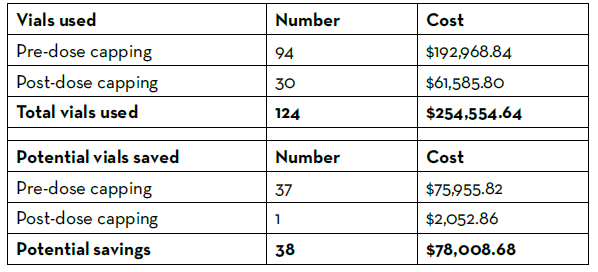
A medication use evaluation was performed to evaluate potential cost savings and safety events associated with the more stringent dose minimization protocol implemented by the factor stewardship committee. Doses of recombinant factor VIIa that were administered between January 1 and December 31, 2021 were included in the study. Patients with hemophilia with documented inhibitors were excluded from the evaluation, as the dose cap for these patients remained at 8 mg. A total of 46 patients with 103 recombinant factor VIIa product administrations were evaluated over the study period, with 29 of the 103 doses being administered after the dose minimization protocol was updated.
Prior to the dose minimization protocol update, 9% of doses exceeded 2 mg, and 47% of doses were between 1 mg and 2 mg. After the dose minimization protocol update, 3% of doses exceeded 1 mg. The number of recombinant factor VIIa vials used over the course of the year were tallied and recorded (Table 1). There were 124 vials used over the evaluation time period, costing the pharmacy department $254,555 using the estimated GPO cost of $2,052.86 per 1 mg vial. Thirty-eight vials could have been saved from use had the doses followed the stricter dosing protocol, leading to an estimated 1-year potential cost savings of $151,912 annually. No life-threatening safety events occurred in patients after the dose minimization protocol was updated. The medication use evaluation concluded that a maximum recombinant factor VIIa dose of 1 mg in non-hemophilia patients and hemophilia patients without inhibitors appeared to be safe and effective in the pediatric population and resulted in significant cost savings with improved revenue integrity.
Summary
Factor stewardship remains an integral effort across healthcare systems to ensuring appropriate use of these high-priced products. Although stewardship strategies have proven effective at other institutions, published guidance for a pediatric-specific program is not available.1-4 The nine years of experience at Norton Children’s Hospital has yielded an overall annual cost savings of approximately 90% since its inception from the approximately $5.2 million annual initial spending in 2013 to approximately $600,000 annual spending in 2020.
Key interventions contributing to the success of our program include dose minimization, vial size rounding, formulary streamlining, and consideration of a consignment-based ordering system.
Table 1: Recombinant Factor VIIa Vials Used and Potential Savings
Future Directions
Future directions for our program include expansion of stewardship efforts into the outpatient setting, which is challenged by the need for patient-specific factor product administration for pharmacokinetic data. The extension of stewardship efforts is aided by the development of newer subcutaneously administered outpatient therapies, such as emicizumab, that reduce the need for regular intravenous factor infusions in certain populations, including hemophilia patients with inhibitors. With further advanced therapies on the horizon and with the continually changing landscape of healthcare, continual quality improvement is necessary to achieve the best possible outcomes for our patients while promoting stewardship of resources.
REFERENCES
- Dane K, Streiff M, Lindsley J, et al. The Development and Impact of Hemostatic Stewardship Programs. Hematol Oncol Clin N Am (2019) 33:887–901.
- Trueg A, Lowe C, Kiel P, et al. Clinical Outcomes of a Pharmacy-Led Blood Factor Stewardship Program (2017) 24:643-647.
- Waheed A, Fongemie J, Gopal S, et al. Implementation and Impact of a Multidisciplinary Coagulation Factor Stewardship Program at an Academic Medical Center. Journal of Thrombosis and Thrombolysis (2020) 50:715–717.
- Amerine L, Chen S, Daniels R, et al. Impact of an Innovative Blood Factor Stewardship Program on Drug Expense and Patient Care. Am J Health- Syst Pharm (2015) 72:1579-84.
Quality Measures: Q&A with Pharmacy Quality Alliance
Ann Schwemm, PharmD, MPH, BCOP
Associate Director, Clinical Oncology
Flatiron Health, Inc
New York, NY
Ben Shirley, CPHQ
Director of Performance Measurement
Pharmacy Quality Alliance
Alexandria, VA
Quality measures are tools commonly used in healthcare today to help quantify various processes, outcomes, and patient perceptions. The Pharmacy Quality Alliance (PQA) is central to medication-related quality measures, which are often adopted by organizations, such as the Centers for Medicare and Medicaid (CMS) and integrated into value-based or quality-based payment programs.
Below, Ben Shirley, CPHQ, from PQA shares a bit about the organization and the processes around measurement development.
Can you share what the Pharmacy Quality Alliance (PQA) is and a bit about your role?
The PQA is a national quality organization dedicated to improving medication safety, adherence, and appropriate use. A measure developer, researcher, educator and convener, PQA’s quality initiatives support better medication use and high-quality care. PQA members include pharmacies, payers, providers, life sciences companies, academics, vendors, and more.
My role at PQA is Director of Performance Measurement, and I’ve been with PQA for about two and a half years. My work pertains to all things quality measures, including development of new measures, maintenance of endorsed measures, providing technical assistance to measure users, and supporting the implementation of PQA measures in quality programs like the Medicare Part D Star Ratings and the Health Insurance Exchange Quality Rating System.
Can you highlight some measures PQA has worked on and how they might impact oncology pharmacists?
PQA’s measure portfolio includes 32 health plan and 10 pharmacy measures. Most recently, PQA developed a standardized Specialty Pharmacy Turnaround Time (SP-TAT-PH) measure, which was endorsed in December 2021. This measure includes a medication list composed of specialty therapies across various categories. One of the categories includes oral oncolytics. The measure can be used by oncology pharmacists to understand their average turnaround time for new prescriptions for these products and can serve as a tool to assess progress when implementing quality improvement initiatives focused on optimizing turnaround time.
Later this year, PQA will convene national leaders to inform and prioritize PQA’s oncology-focused quality and research strategies, including opportunities for pharmacists and other providers to improve oncology medication use quality.
Can you share, at a high level, what it means that PQA is a “measure developer”?
Simply put, measure developers are organizations that create quality measures. As the healthcare system has increasingly shifted from paying for volume to paying for value, the importance of metrics that can accurately and fairly assess quality of care has grown considerably. Measure developers are specialized organizations that combine expertise in clinical care, measurement science, statistics, program management and population health to develop rigorous quality measures that meet high standards.
PQA’s development process also integrates patient partners to ensure the patient voice and preferences are reflected in quality measurement. Because of PQA’s multi-stakeholder, consensus-based measure development process, once PQA measures are endorsed by our membership, they are suitable for their intended use in the marketplace and can be adopted into various programs. Visit our measure-development page at pqaalliance.org to learn more.
It’s important to note that PQA is not only a measure developer, but also a measure steward. After developing and endorsing measures, PQA uses a systematic, consensus-based maintenance process to ensure those measures continue to align with current evidence and guidelines and remain relevant over time. As a steward, we support use of our measures and work closely with program administrators to ensure accurate implementation. We also provide technical support to measure users, create educational content and webinars, and more.
What key characteristics does PQA consider when developing a measure?
PQA uses standard measure criteria to evaluate measures, which closely align with criteria used by other organizations such as the National Quality Forum and CMS in the Measures Management System Blueprint.
The first criterion is importance, which looks at how important a given concept is to measure and report. For example, is the measure evidence based? What is the size of the patient population included in the measure, and to what extent are those patients affected by the processes or outcomes assessed in the measure? Is there a gap in performance such that there’s opportunity for improvement?
The second criterion is scientific acceptability, which is broken down into two concepts: validity, which tells us if we’re truly measuring what we intend to measure, and reliability, which tells us if our measurements are consistent, and whether differences in score are due to quality or just due to statistical noise or chance. These questions are answered through the measure testing process and through expert input.
The third criterion is feasibility. A measure may be important, valid and reliable, but if we can’t measure it due to data availability, it doesn’t do us much good. Feasibility addresses whether we have the tools, the data, and the reporting infrastructure to implement the measure without introducing too much burden.
The final criterion is usability. It’s helpful to think of this as if we build it, will they come? Is there an opportunity for implementation in programs or contracts? Are the results meaningful to the users of the measure, and can they be used to drive improvement? Finally, are there any concerns about unintended consequences?
What do you recommend those using the measures for quality improvement consider?
Accurate measurement is a critical part of any quality improvement initiative. Many quality improvement initiatives use a “plan-do-study-act” (PDSA) approach to evaluate interventions. Without appropriate measurement, the ‘study’ portion of the PDSA cycle will not be successful. Therefore, using quality measures to set targets and track progress towards those targets can be an excellent way to improve quality over time.
The measures created by PQA are often integrated into various quality or value-based payment programs. What process does PQA go through to prioritize measures to develop?
Deciding which new measures to develop, especially given a finite amount of time and development resources, is an important consideration for PQA. To that end, the first stage of the PQA measure development lifecycle is conceptualization, which focuses on systematically prioritizing measure concepts for development.
The conceptualization process begins with environmental scanning and gap analyses that evaluate measurement needs in current programs. For example, a recent round of conceptualization identified a lack of health plan measures focused on the chronic obstructive pulmonary disease (COPD) population. Following the environmental scan, potential measure concepts are shared with and discussed by a Measure Concept Advisory Group (MCAG). The MCAG is a group of experienced PQA members representing a diverse mix of stakeholders who are charged with evaluating potential measure concepts based on criteria like importance, feasibility, and usability to advise PQA on priorities.
Measure concepts recommended for development by the MCAG are then released by PQA for a round of public comment to gain further insight. Based on public comments, MCAG input, and staff deliberation, PQA finalizes priorities, launches Technical Expert Panels for chosen concepts, and begins the specification process.
Is there anything else in the measure development process our readers should know?
Developing measures takes time! The timelines can vary based on the complexity of the measure, but 18-24 months is a common end-to-end development for a relatively straightforward measure. Whether it’s initial conceptualization, convening and meeting with our expert panels, the testing process, or achieving PQA-endorsement through our consensus-based process, there’s a lot of work that goes into creating a quality measure.
Beyond development, implementing measures into programs also takes time. For example, a measure being added to the Medicare Part D Star Ratings might start out in the Patient Safety Reports for health plan internal quality improvement. If a measure is being considered for Star Ratings, it would be added to the Display Page for at least two years, and then go through the rulemaking process before moving into the Star Ratings. All in all, the process can take quite a long time.
Can you share with us considerations related to current medication adherence measures and why they may not be able to be directly applied to oral anticancer agents?
PQA’s adherence measures are calculated using prescription claims data based on the proportion of days’ covered (PDC) methodology. This approach has been empirically validated, and use of the PDC measures has been associated with substantial impacts on patient health and cost reductions in reports like the CMS National Impact Assessment.
The PDC methodology may not be appropriate for assessing adherence to certain medication classes. For example, it is not possible to discern the rationale for medication discontinuation using prescription claims data. For that reason, it’s important to only include medication classes expected to be used on an ongoing basis. This can introduce challenges with oral oncolytics given the possibility for decreased dosage or discontinuation due to toxicity. Another important aspect of PDC measures is a clear evidence base for the medications included in the measure. Oral oncolytics have a highly complex evidence base that varies across specific regimens, which can introduce substantial complexity with fairly assessing adherence when individuals switch among different regimens.
In internal evaluations and in external groups and task forces PQA has participated in, adherence or persistence to oral oncolytics has been noted to be quite challenging for measurement due to these considerations, and additional pre-development analyses and research may be needed to inform potential development. Other areas, such as provider-patient communication or screening for medication access challenges have been raised as potential alternatives.
Novel Therapies in Sickle Cell Disease: Hype or Hope?
Madeleine Ochs, PharmD
Clinical Pharmacist Specialist, Inpatient Hematology
Michigan Medicine: University of Michigan
Ann Arbor, MI
Sickle cell disease (SCD) is an inherited red blood cell disorder, impacting roughly 100,000 people in the United States. SCD is caused by a single nucleotide substitution in the sixth codon of the beta-globin gene, which results in the substitution of valine for glutamic acid and the production of sickle hemoglobin (HbS). This substitution allows HbS to polymerize under deoxygenated conditions, distorting red blood cells into a sickled shape.1 Since the United States Food and Drug Administration (FDA) approval of hydroxyurea in 1998, the armamentarium for the treatment of SCD has been limited.
Although its mechanism is not fully understood, hydroxyurea is a ribonucleotide reductase inhibitor and increases synthesis of fetal hemoglobin. Fetal hemoglobin, which lacks beta-globin chains, inhibits sickling by interfering with the polymerization of HbS. The Multicenter Study of Hydroxyurea demonstrated that hydroxyurea significantly reduced the median annual rates of crises compared to placebo (median 2.5 vs 4.5 crises, p<0.001) in adults with ≥3 crises in the previous year.2 Long-term follow-up after 9 years found that hydroxyurea demonstrated a 40% reduction in mortality.3 Despite the mortality benefit, limitations with hydroxyurea include toxicity (myelosuppression, GI symptoms, infection, secondary malignancies) and poor adherence rates, partially due to patient perceptions and misconceptions.
Novel Therapies
Whereas there was only one approved treatment option over the last two decades, from 2017-2019 we saw a relative explosion of FDA approvals for the treatment of SCD, with 3 drugs approved: L-glutamine, crizanlizumab, and voxelotor (Table 1).
L-glutamine
L-glutamine is a conditionally essential amino acid, which is required for the synthesis of NADPH, glutathione, and nitric oxide and becomes essential during times of oxidative stress, as in SCD.4 In 2017, L-glutamine received FDA approval based on the results of a phase III trial comparing L-glutamine 0.3 g/kg/dose by mouth (PO) twice daily (BID), max 30 g/day, to placebo in patients age ≥5 years who had ≥2 pain crises in the prior year.5 Patients were eligible if they were receiving hydroxyurea at a stable dose for ≥3 months, although hydroxyurea couldn’t be initiated or dose-escalated during the study. Roughly 2/3 of patients in both arms received concomitant hydroxyurea. Exclusion criteria included receipt of any blood products within three weeks prior to screening. The primary endpoint—the number of pain crises [defined as pain requiring parenteral narcotics or ketorolac in the ED/during hospitalization, acute chest syndrome (ACS), priapism, or splenic sequestration] through week 48—was significantly lower in the L-glutamine arm, with a median of 3 pain crises with L-glutamine vs 4 with placebo, p=0.005. L-glutamine also resulted in fewer hospitalizations (median 2 vs 3, respectively), but there was no difference in ED visits between arms. The discontinuation rate was higher in the L-glutamine arm at 36.2%, compared to 24.4% with placebo. The poor adherence rates with L-glutamine are unsurprising as it is supplied as a 5 g/packet oral powder, which is mixed with 240 ml of a cold/ room temperature beverage or 120-180 ml of food.6 Patients ≥65 kg receiving 15 g BID are therefore required to mix 3 packets two times daily. Because of the high dropout rate, the trial investigators imputed the pain crises as the mean of the patients in the same arm or the actual number of crises at the time of discontinuation carried forward for the duration of the 48 weeks, whichever was higher.
Due to the limitations of the statistical analyses, the FDA conducted their own sensitivity analyses and estimated that L-glutamine reduced the mean number of sickle cell crises from 0.4-0.9, compared to a difference of 1 in the published analysis. This study did not evaluate quality of life (QOL). Overall, L-glutamine was well tolerated; nausea, extremity pain, and back pain were more common in the L-glutamine arm. Despite the 0.3 g/kg twice daily dosing regimen studied in the phase 3 trial, the FDA approved dosing strategy is based on weight ranges (<30 kg: 5 g BID; 30-65 kg: 10 g BID; >65 kg: 15 g BID).
Crizanlizumab
Crizanlizumab is a humanized IgG2 kappa monoclonal antibody that binds P-selectin and prevents its interaction with P-selectin glycoprotein ligand 1 (PSGL-1), thereby decreasing the adhesion of sickled red cells.7 Crizanlizumab was FDA approved based on the results of the phase 2 SUSTAIN trial, which randomized 198 patients with SCD to low dose crizanlizumab (2.5 mg/kg), high dose crizanlizumab (5 mg/kg), or placebo IV every 2 weeks for 2 doses, followed by every 4 weeks for a total of 14 doses.8 Trial participants were 16-65 years old with 2-10 pain crises in the prior year. Again, patients receiving hydroxyurea were required to be at a stable dose for ≥3 months and hydroxyurea couldn’t be initiated or dose-escalated during the study. Patients receiving chronic transfusions were excluded. The primary endpoint, the median annualized rate of sickle cell pain crises (defined as acute episodes of pain resulting in treatment with PO/IV narcotics or IV NSAIDS at a medical facility, ACS, hepatic or splenic sequestration, or priapism) was significantly lower with high dose crizanlizumab at 1.63 vs 2.98 with placebo, p=0.01. There was no significant difference in the annual rate of days hospitalized, median rate of complicated crises including ACS, or QOL between arms. Again, due to the high discontinuation rate (35.8% high-dose, 31.8% low-dose, 36.9% placebo) the FDA performed sensitivity analyses.9 The decreased annual rate of vaso-occlusive crises (VOC) with high-dose crizanlizumab maintained significance when patients who discontinued the study early were excluded (1.18 vs 2.98 with placebo, p=0.005), however significance was lost when including only those who discontinued early.
Overall, crizanlizumab was well tolerated, and the most common adverse events in the high-dose group included nausea (18%), arthralgia (18%), headache (17%), extremity pain (17%), and back pain (15%). Serious adverse events (grade ≥3) in either crizanlizumab arm included pyrexia (n=2), influenza (n=3), and pneumonia (n=5). Infusion related reactions (occurring up to 24 hours post-infusion) occurred in 2 patients, and premedication with acetaminophen and diphenhydramine may reduce these reactions. A negative pregnancy test was required prior to initiation of crizanlizumab during the trial and incorporating pregnancy tests into order sets can help ensure negative pregnancy tests prior to treatment.
During the SUSTAIN trial, no antibodies against crizanlizumab were detected, however post-marketing studies required by the FDA are ongoing. Because crizanlizumab interferes with automated platelet counts resulting in platelet clumping when blood samples are collected using EDTA tubes, citrate tubes should be used to collect blood samples.7 Based on the results of the SUSTAIN trial, the FDA approved dose of crizanlizumab is 5 mg/kg.
Voxelotor
Voxelotor is a HbS polymerization inhibitor, which binds reversibly to hemoglobin and increases the affinity of hemoglobin for oxygen. Voxelotor was FDA approved in 2019 as a result of the phase 3 HOPE trial, which randomized patients to voxelotor 1500 mg or 900 mg PO once daily, or placebo.10 Eligible patients with SCD were age 12-65 years, with a hemoglobin of 5.5-10.5 g/dL, and had 1-10 VOCs in the prior 12 months. Similar to previous trials, hydroxyurea use was permitted if patients were on a stable dose for ≥3 months. Overall, 65% of patients were receiving hydroxyurea at baseline. Patients receiving chronic transfusions or those who received a transfusion in the previous 60 days were excluded. The primary endpoint was the percentage of patients with an increase in baseline hemoglobin by ≥1.0 g/dL at week 24, in contrast to the prior L-glutamine and crizanlizumab trials where pain crises were the primary endpoint. At week 24, voxelotor 1500 mg resulted in significantly more patients with a hemoglobin response compared to placebo (51% vs 7%, p<0.001; 33% with 900 mg voxelotor). With long-term follow-up, 89% of patients in the 1500 mg voxelotor arm, 72% with 900 mg, and 25% with placebo had an increase in hemoglobin by ≥1.0 g/dL at any time point by week 72.11
Despite the improvement in hemoglobin, there was no difference in the percentage of patients requiring RBC transfusions during the study, at 36% in all arms. Similarly, there was no significant difference in the annualized rate of VOCs (defined as pain requiring IV/PO opioids, ketorolac, or other analgesics or ACS) at 2.4 with voxelotor 1500 mg, 2.4 with 900 mg, and 2.8 with placebo. Based on a subgroup analysis, the authors claimed the incidence of VOCs was lowest among patients with the highest hemoglobin, ≥12 g/dL (n=10). Sickle cell anemia with crisis, ACS, priapism, and osteonecrosis were defined as sickle cell related events in contrast to the composite endpoint in the prior studies. There was no difference in the rate of sickle cell related adverse events among arms (78% 1500 mg, 75% 900 mg, 80% placebo), and grade 3 events were numerically higher in the 1500 mg arm (priapism: 6.5% 1500 mg, 2.4% 900 mg, 2.4% placebo; ACS 9.1% 1500 mg, 8.7% 900 mg, 6.6% placebo). In comparison to the previously discussed trials, the discontinuation rate was lower at 26% in the voxelotor 1500 mg arm, which may be attributed to the once daily dosing regimen. In the high dose voxelotor arm, the most common adverse events were headache (26%), diarrhea (20%), nausea (17%), and arthralgia (15%).
Voxelotor is a minor CYP3A4 substrate and weak CYP3A4 inhibitor and dose adjustments are recommended for use with concomitant CYP3A4 inducers or inhibitors.12 Based on the HOPE trial, the FDA approved dose of voxelotor is 1500 mg once daily. Despite PK studies with voxelotor 600 mg daily demonstrating mean AUC levels (concentrations adjusted for dose) were 90% higher with severe hepatic impairment, the package insert recommends dose adjustment to 1000 mg for severe hepatic impairment.13 Voxelotor may interfere with measurement of Hb subtypes and if needed, chromatography should be performed when patients are not receiving voxelotor.
Hype or Hope?
While it is encouraging there are now additional drug treatment options beyond hydroxyurea, the definition of acute pain crises, primary endpoints, and baseline characteristics differ across studies, which makes it difficult to determine the optimal place in treatment for these novel agents. The variable use of hydroxyurea and the inability to initiate or dose escalate hydroxyurea in the trials makes it challenging to determine what patients will benefit most from these novel therapies. The benefit of these agents in patients receiving chronic transfusions is unknown, as they were excluded from these trials. Furthermore, there is no long-term follow-up data available for these novel agents. There is no safety data in pregnancy as pregnant patients were excluded in all three trials. Few pediatric patients were included in these studies, with a minimum age of 5 years for L-glutamine, 12 years for voxelotor, and 16 years for crizanlizumab, however pediatric studies are ongoing.
Voxelotor recently received accelerated approval for use in children ages ≥4 years based on the Phase 2 HOPE-KIDS Study. Among the trials which examined QOL, there was no improvement demonstrated (not studied for L-glutamine). In addition to the limitations discussed above, these novel agents are associated with significant financial toxicity. Based on wholesale acquisition costs, a 30-day supply costs roughly $3,690 for L-glutamine, $9,642 for crizanlizumab, and $10,417 for voxelotor. This is especially relevant as these costs are likely prohibitive for patients who live in countries where the SCD burden is the highest.
As hydroxyurea has a demonstrated mortality benefit, these novel agents should not be used as a replacement for hydroxyurea, but rather in addition to hydroxyurea for patients with inadequate disease control. Instead, pharmacists can play a large role in helping increase adherence to hydroxyurea. L-glutamine and crizanlizumab could be considered for patients with ≥2 VOCs in the prior year and who are intolerant to or have a contraindication to hydroxyurea. However, the benefit of L-glutamine was impacted by the imputation method utilized to account for the high discontinuation rate. The role of voxelotor, if any, is limited to patients with symptomatic anemia but should be used with caution as it did not demonstrate any benefit on transfusion burden, VOCs, or QOL.
While there is a lot of hype with the novel therapies recently approved, I am hopeful for the number of investigational drugs for SCD in the pipeline. Allogeneic hematopoietic cell transplantation is the only curative option for SCD; however, its use is limited by the availability of suitable donors. Gene therapy is another potentially curative option and recent data demonstrated complete resolution of VOCs in patients who had ≥4 severe VOCs in the prior 2 years.14 Additional emerging therapies include incalcumab and GBT021601, which share mechanisms with crizanlizumab and voxelotor, respectively, but are administered at a reduced frequency/pill burden and agents with novel mechanisms such as compliment inhibition.
Table 1. Summary of Novel Agent Clinical Trials in Sickle Cell Disease
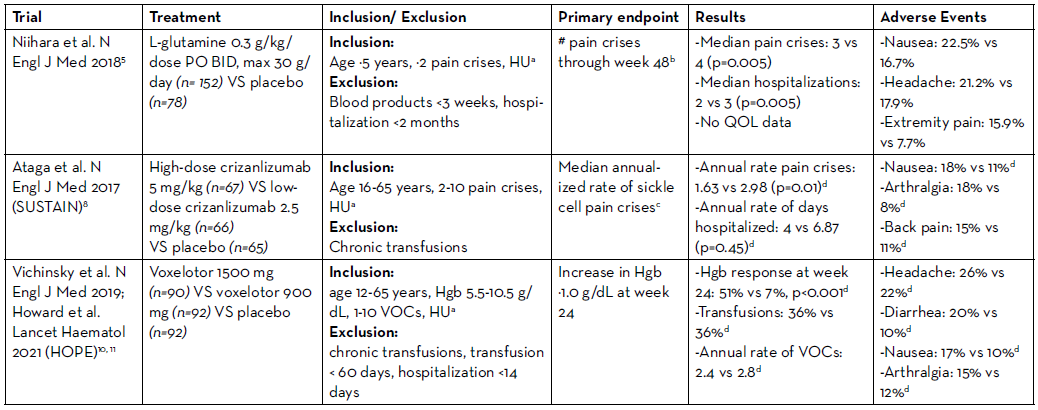
HU: hydroxyurea; Hgb: hemoglobin
aConcomitant hydroxyurea permitted if at a stable dose for ≥3 months; no initiation or dose-escalation allowed
bPain requiring parenteral narcotics or ketorolac in the ED/during hospitalization, ACS, priapism, or splenic sequestration
cAcute episodes of pain resulting in treatment with PO/IV narcotics or IV NSAIDS at a medical facility, ACS, hepatic or splenic sequestration, or priapism
dHigh-dose vs placebo
REFERENCES
- Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004 Oct 9-15 2004;364(9442):1343-60. doi:10.1016/S0140-6736(04)17192-4.
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. May 18 1995;332(20):1317-22. doi:10.1056/NEJM199505183322001.
- Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. Apr 02 2003;289(13):1645-51. doi:10.1001/jama.289.13.1645.
- Sadaf A, Quinn CT. L-glutamine for sickle cell disease: Knight or pawn? Exp Biol Med (Maywood). 01 2020;245(2):146-154. doi:10.1177/1535370219900637.
- Niihara Y, Miller ST, Kanter J, et al. A Phase 3 Trial of l-Glutamine in Sickle Cell Disease. N Engl J Med. Jul 19 2018;379(3):226-235. doi:10.1056/ NEJMoa1715971.
- Endari. Package insert. Emmaus Medical I, 2017.
- Adakveo. Package insert. Novartis Pharmaceutical Corporation; 2019.
- Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N Engl J Med. 02 02 2017;376(5):429-439. doi:10.1056/NEJMoa1611770.
- Center for Drug Evaluation and Research. BLA Multi- Disciplinary Review and Evaluation, BLA 761128 ADAKVEO (Crizanlizumab-tmca). Accessed March 1, 2022 https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761128Orig1s000MultidisciplineR.pdf.
- Vichinsky E, Hoppe CC, Ataga KI, et al. A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease. N Engl J Med. 08 08 2019;381(6):509-519. doi:10.1056/NEJMoa1903212.
- Howard J, Ataga KI, Brown RC, et al. Voxelotor in adolescents and adults with sickle cell disease (HOPE): long-term follow-up results of an international, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Haematol. May 2021;8(5):e323-e333. doi:10.1016/S2352- 3026(21)00059-4.
- Oxbryta. Package insert. Global Blood Therapeutics I, 2019.
- Preston RA, Marbury T, Balaratnam G, et al. Pharmacokinetics of Voxelotor in Patients With Renal and Hepatic Impairment. J Clin Pharmacol. 04 2021;61(4):493-505. doi:10.1002/jcph.1757.
- Kanter J, Walters MC, Krishnamurti L, et al. Biologic and Clinical Efficacy of LentiGlobin for Sickle Cell Disease. N Engl J Med. 02 17 2022;386(7):617- 628. doi:10.1056/NEJMoa2117175.
Best Practices for Research Manuscripts in Two Parts: How to Pursue Publication and How to Become a Peer Reviewer
Christina Howlett Fullerton, PharmD, BCOP
Clinical Director, Research Oncology - Real World Evidence
Flatiron Health
New York, NY
Scott A. Soefje, PharmD, MBA, BCOP, FCCP, FHOPA
Director, Pharmacy Cancer Care
Mayo Clinic
Rochester, MN
There are many areas of uncertainty that may arise when developing a research manuscript for publication. Here are some tips for navigating the publication process with confidence and ease, followed by best practices for becoming a peer reviewer.
Research Focus: How to Pursue Publication
Find a Target Journal
In developing your manuscript, it is very helpful to have your target journal in mind from the beginning. By incorporating the journal’s style requirements for submission into your early drafts, you are avoiding having to do major revisions to meet their requirements at the time of submission. Locating journals that encompass the genre of the research topic and confirming that the journal has published research with similar study designs is critical.
It is also helpful to read the journal’s recently published articles to determine if your study aligns well with the current goals of the publication. If you are setting out to write a review article or case report, it may be helpful to reach out to the editor and inquire if there are any themed publications on the horizon for which your submission may be of interest. This can help with directing a case report or review article toward a specific goal for submission.
Create a Timeline & Seek Feedback Often
To stay on track, like any other project, it is best to keep a timeline for your manuscript from development through submission and peer-review. Having all team members involved and engaged throughout the process is critical for success, and this includes timeline execution.
If you are the resident on the project, you can decide how best to allow for editing by your mentors. Seeking out feedback early and often can allow for utilization of this guidance for future iterations. Also, writing a section at a time and asking for feedback on that section before moving to the next can allow for a more continuous process of writing and editing (i.e., You ask for feedback on the Background section and while mentors work on providing feedback for this section, you start writing the Methods section).
The most critical piece of advice regarding publication submission of residency research projects is to keep the process moving after residency graduation. Whether you are still in the editing phase, or getting the manuscript tidied up before submission, keeping up with these final tasks are critical for getting to the finish line. This may also be the time you hear back from the journal that they are accepting, accepting with minor/major revisions or are not accepting your manuscript for publication. If the latter is the case, do not fret, you can try again! Finding a new target journal and making edits according to the new journal’s style and prior journal’s feedback understandably do take time but stay the course. It may take 2-3 submissions to find the right fit.
Utilize What you Have
Keep in mind that you have already written early draft sections of your manuscript throughout the research project process. Even though your IRB Background section may need substantial work when translating it into the manuscript Background, it is a great place to start. You also have the Background references from the section pulled and possibly a conference abstract written. These written pieces can all contribute to your manuscript development, so do not let them go to waste. Even just having them as a placeholder for future editing can be reassuring when you set out to write your manuscript.
Network, Network, Network
As a general piece of advice for moving research projects to publication, creating a professional network of co-researchers is enormously helpful. As you move from residency to a full-time clinical role, there may be a variety of novel research ideas you come across. Keep discussing these ideas with pharmacy colleagues, as well as multidisciplinary team members. This will spark new research projects, allow for collaborations across disciplines, and foster opportunities for publishing in other healthcare discipline journals. Also, moving into your clinical role will find you on the preceptor side of resident research and publishing. Acting as a residency research advisor can help to expand your own research methodology skills and allow you to be better equipped in responding to feedback during the journal peer-review process.
It is a very rewarding process and an honor to contribute to the breadth of peer-reviewed research used every day in patient care.
Research Focus: How to Become a Peer Reviewer
Doing peer reviews for journals can be an interesting, professionally rewarding activity. Peer reviews can help you become a better writer and researcher while giving you a glimpse into the publication process. It helps build your reputation as it sets you up as an expert. Most importantly, it improves the quality of the research process by preventing the publication of poorly done research and helps select papers that will be of most interest to readers.
How to Become a Peer Reviewer
One question is “how do I become a peer reviewer?” Journals are looking for professionals that are experts in their area. Often the journals will look for people that have published on the topic the article is discussing. Networking is another way, so if you know someone on the editorial board of a journal, ask them how to become a peer reviewer. The last way is to contact the journal and the editors directly. Explain your expertise, express your areas of interest, and your desire to review articles. Good peer reviewers will be asked to do more reviews and sometimes start getting requests from other journals.
When to Accept—and When to Decline—an Invitation to Review
When the request to peer review arrives in your inbox, take the time to review the information. Make sure you can meet the requested deadline. If you want to review the paper but cannot meet the deadline, ask for an extension as it is possible the journal may make your timeline work for their publication. It is often easier for the editor to give you an extension than try to find another reviewer. It is perfectly acceptable to decline the review because it is better to decline an offer than to do a poor review. If you decline, be prompt in your response, explain why you are declining, and, if possible, recommend an alternative reviewer that may be better suited for the review. Here you can take the opportunity to express that you would be willing to do reviews, tell them what your strengths are, and that you would like to see other opportunities.
Critical Reading and Commenting
If you decide to accept the peer review offer, read the journal guidelines and scope, since these instructions were made to guide you on how to do the most useful review. It is recommended that you read the paper multiple times. The first reading should be to get the overall impression of the article. Does it fit the scope of the journal? Is there a fatal flaw that stops the publication right there? Is this a paper you found interesting to read? The next reading should concentrate on the scientific aspects of the paper. Is the abstract a clear overview of the work? Does the introduction explain the rationale? Are the methods sound and the results present well with a complete analysis? Do the conclusions accurately describe the data? Either as a final reading or during the first two readings, concentrate on writing and presentation of the paper. DO NOT line edit the paper—the editorial staff will fix the grammar and spelling issues later. Focus on the bigger picture items such as, too many grammar and spelling mistakes, hard to read, or does not flow well. You are looking for readability and style.
Be Thorough and Balanced
You will be asked to make two sets of comments. One will go to the authors that are designed to make the paper better. List out the flaws in the paper and what the author should do to address the flaws. Be kind, but honest. Be concise and specific. You are outlining for the author what needs to be done to make the paper publishable.
The second set of comments goes to the editor and will not be seen by the author. Here you will usually make the recommendation acceptable as written (which rarely happens), acceptable with minor revisions, acceptable with major revisions, or reject the paper. If you recommend revisions, describe which items must change to make the paper acceptable and which are nice to change. Do not be afraid to reject a paper but do tell the editor the rationale for why you think the paper should be rejected. The best thing you can do as a reviewer is to outline the paper’s benefits and issues honestly and clearly as you see them and share the specifics as to why you see them that way.
The best reviews are thorough and balanced, and the absolute best reviewers provide such comments in a timely manner. Peer review is a collaboration that improves the overall quality of publications that you can find is a professionally satisfying activity.
REFERENCES
- Kostic M. How to be a Great Reviewer for a Research Paper. Cell Mentor. Aug 31, 2017. Available at http://crosstalk.cell.com/blog/how-to-be-a-great-reviewer-for-a-research-paper. Accessed 3/18/22.
- McPeek MA, DeAngelis DL, Shaw RG, et.al. The Golden Rule of Reviewing. Am Nat. 2009;173(5):E155-8
- Stiller-Reeve M. How to Write a Thorough Peer Review. Nature Career Column. Oct 8, 2018. Available at https://www.nature.com/articles/d41586- 018-06991-0. Accessed 3/18/22.
- Stiller-Reeve M. A Peer Review Process Guide. The Scholarly Kitchen. April 2018. Available at https://www.scisnack.com/?s=peer+review+process. Accessed 3/18/22.
- Wallace J. How to be a Good Peer Reviewer. The Scholarly Kitchen. Sep 17, 2019. Available at https://scholarlykitchen.sspnet.org/2019/09/17/how-to-be-a-good-peer-reviewer/. Accessed 3/18/22.
Feature: The Role of Tumor-infiltrating Lymphocytes (TILs) in the Treatment of Melanoma
Colleen McCabe, PharmD, BCOP
Clinical Pharmacy Specialist - Sarcoma/Melanoma
Vanderbilt University Medical Center
Nashville, TN
Jordyn Higgins, PharmD, BCOP
Clinical Assistant Professor of Pharmacy Practice in Oncology
Mercer University College of Pharmacy
Clinical Pharmacy Specialist- Medical Oncology
Emory Winship Cancer Institute
Atlanta, GA
The Immune System and Melanoma
In addition to protecting against external pathogens, the human immune system has evolved to produce defense mechanisms against a variety of diseases, including cancer.1 One such mechanism is the process called immunological surveillance, which monitors internal cell structure to recognize and destroy cancer cells. The human immune system plays a large role in the defense against melanoma in particular.2 Melanoma is widely recognized as one of the most immunogenic types of cancer and is the fifth most common cancer type in the United States.3
Although the human immune system employs many mechanisms to detect melanoma cells, melanoma cells employ numerous mechanisms to avoid detection. These mechanisms include downregulation of targetable molecules and elaboration of immunosuppressive cytokines. Therefore, the immune system alone is largely inadequate to fight off melanoma cells. However, understanding the immune mechanisms of melanoma cells offers an exciting opportunity for drug development and improved treatment modalities.2
Recent drug development has focused on exploiting the immunogenic nature of melanoma to develop effective therapies for treatment. This is particularly noticeable in the metastatic disease treatment setting, which heavily utilizes immune checkpoint inhibitors. This article explores the role of a specific immune-cell tumor-infiltrating lymphocytes (TILs)—and the role these cells play in disease prognosis and treatment.
Tumor-infiltrating Lymphocytes
TILs are a polymorphic group that is composed mainly by effector T lymphocytes, regulatory T lymphocytes, natural killer cells, dendritic cells, and macrophages.4 TILs can recognize cancer cells as abnormal, penetrate the tumor, and kill cancer cells.5 TILs are lymphocytes present within tumors, and in the case of primary melanoma, they are found alongside melanoma cells. TILs are classified as absent, nonbrisk, or brisk. Absent TIL indicates no lymphocytes directly opposed to tumor cells; nonbrisk TIL indicates the presence of isolated, multifocal, and segmental TIL infiltrate in the tumor, and brisk TIL is defined as the infiltration of the entire base of the tumor or diffusely meeting the tumor.6
The Relationship Between TILs and Regression in Melanoma
Lymphocyte infiltration of melanoma is a widespread response of the host immune system to the presence of tumor cells. Histologic regression is defined as the replacement of tumor cells with immune cells, melanin-laden macrophages, and fibrotic components.7 Immune cell infiltration of the cancer cells is a result of the host cells recognition of cancer cells and immunologic response as a defense mechanism.8 Studies have supported that histologic regression is associated with improved survival.7
Historically, the classification of melanoma stage is based on pathological features of the primary tumor and the spread of the disease at the time of diagnosis. Staging is a major contributor to treatment decision-making. As the level of understanding of the immunogenicity of the melanoma disease state grows, numerous studies have provided evidence to include immune system biomarkers in the staging of melanoma. A large meta-analysis of the data in 2020 supported the use of TIL level in prognostic criteria as they found the published literature to show brisk TIL grade had a better prognosis.8
In particular, TILs elicit immunity locally and may decrease the likelihood of sentinel lymph node (SLN) metastases. Interestingly, both TIL presence and differentiation and localization have been shown to determine clinical outcomes.9 TIL infiltration is manually quantified by pathological assessment; however, digital immune scores, which may provide the opportunity to translate the prognostic benefit of TILs into a clinically usable diagnostic tool, are still under development.9 Therefore, the presence of TILs is a marker of immune activity and a favorable biomarker.7 In fact, TILs have been associated with improved survival.10–13 In addition to its incorporation into staging guidelines, TIL presence can also be exploited for the treatment of melanoma. In fact, the National Comprehensive Cancer Network (NCCN) guidelines encourage utilization in prognosis determination in addition to Breslow thickness and other primary lesion characteristics.14
The Future Role of TILs in Treatment of Melanoma
TILs are an experimental cell therapy for the treatment of melanoma. Currently, this therapy is only available via a clinical trial for patients who have already received standard of care immunotherapy and BRAF/MEK inhibitors (if applicable). The process of extraction, engineering, and administration to the patient is similar to chimeric antigen receptor-T (CAR-T) therapy. TILs are harvested from the patient and taken to the lab for expansion and engineering. Patients receive a short-term chemotherapy regimen, then the patient receives the TILs through an infusion.
An advantage of TILs compared to CAR-T therapy is that these come directly from the tumor and do not need to be genetically engineered to recognize the tumor since they were innately designed to do so.5 Additionally, the use of CAR-T therapy in solid tumors is limited by organ toxicities. The risk of these toxicities occur due to activation of T cell effector function through the CAR as most tumor-associated antigens are also found in normal tissues.15 Another advantage of TILs is that they are highly polyclonal and do not have as high of a risk of organ toxicities. General side effects seem to be related to the chemotherapy or interleukin-2 (IL-2) if administered in combination with the TILS therapy.
Two TIL therapies show promise in the treatment of melanoma. The United States Food and Drug Administration (FDA) granted orphan drug designation to a novel TIL therapy (ITIL-168) for the treatment of stage IIB to IV melanoma based on the results of a retrospective study of 21 compassionate use patients assessing the feasibility of utilizing TILs for the treatment of advanced cutaneous melanoma. Patients were hospitalized for treatment administration and for side effect management. ITIL-1618 was administered at 600,000-720,000 IU/kg after cyclophosphamide 60 mg/kg/day for 2 days and fludarabine 25 mg/m2/day for 5 days.
In all evaluable patients (n=21), best overall responses were observed in 67% of patients (complete responses: 19%, partial responses: 48%). Median overall survival was 21.3 months (95% Confidence Interval [CI], 6.8-not evaluable). At median follow-up of 52.2 months 23% of the 14 responding patients experienced durable responses.16 The investigational new drug (IND) application was accepted by the FDA for DELTA-1, a global phase II clinical trial of ITIL-168 in patients with advanced melanoma whose disease has relapsed after a programmed death protein 1 (PD-1) inhibitor and if positive for a BRAF-activating mutation, a BRAF inhibitor.
Next, a phase II, open-label, single-arm, multicenter study in patients who had been previously treated with checkpoint inhibitor(s) and BRAF +/- MEK targeted agents was conducted in 66 patients. Patients received a nonmyeloablative lymphodepletion regimen, a single infusion of lifileucel (LN-144), and up to six doses of high-dose interleukin-2. The overall response rate was 36% (95% CI, 25-49). Disease control rate was 80% (95% CI, 69-89) and median duration of response was not reached after the 18.7-month median study follow-up (range, 0.2-34.1 months).17
Conclusion
Melanoma has long been recognized as one of the most immunogenic of cancer diagnoses. Especially in comparison to other tumor types, melanoma has vast opportunities for the development of immunotherapeutic strategies. One marker of the immune system involvement in melanoma is the presence of tumor-infiltrating lymphocytes. Lymphocyte infiltration of melanoma is a widespread response of the host immune system to the presence of tumor cells. Histologic regression is defined as the replacement of tumor cells with immune cells, melanin-laden macrophages, and fibrotic components.7 TILs are still considered an experimental cell therapy for the treatment of melanoma, but clinical trial data has shown promising results for future incorporation into the treatment guidelines.
REFERENCES
- Mukherji B. Immunology of melanoma. Clinics in dermatology. 2013;31(2):156-165. doi:10.1016/J.CLINDERMATOL.2012.08.017.
- van den Berg JH, Heemskerk B, van Rooij N, et al. Tumor infiltrating lymphocytes (TIL) therapy in metastatic melanoma: boosting of neoantigen-specific T cell reactivity and long-term follow-up. Journal for immunotherapy of cancer. 2020;8(2). doi:10.1136/JITC-2020-000848.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69(1):7-34. doi:10.3322/CAAC.21551.
- Antohe M, Nedelcu RI, Nichita L, et al. Tumor infiltrating lymphocytes: The regulator of melanoma evolution. Oncology letters. 2019;17(5):4155-4161. doi:10.3892/OL.2019.9940.
- What is tumor-infiltrating lymphocyte (TIL) therapy? | MD Anderson Cancer Center. Accessed March 22, 2022. https://www.mdanderson.org/cancerwise/what-is-tumor-infiltrating-lymphocyte-til-therapy--6-things-to-know.h00-159460056.html.
- Clark WH, Elder DE, Guerry D, et al. Model predicting survival in stage I melanoma based on tumor progression. Journal of the National Cancer Institute. 1989;81(24):1893-1904. doi:10.1093/JNCI/81.24.1893.
- Morrison S, Han G, Elenwa F, et al. Is There a Relationship Between TILs and Regression in Melanoma? Annals of surgical oncology. Published online 2022. doi:10.1245/S10434-021-11251-Z.
- Sun Q, Sun H, Wu N, Cong L, Cong X. Prognostic Significance of Tumor-Infiltrating Lymphocyte Grade in Melanoma: A Meta- Analysis. Dermatology (Basel, Switzerland). 2020;236(6):481-492. doi:10.1159/000505152.
- Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cellular & Molecular Immunology 2020 18:4. 2020;18(4):842-859. doi:10.1038/s41423-020-00565-9.
- Clark WH Jr E de. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893-1904.
- F Azimi RSPR. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30:2678-2683.
- CG Clemente MMRBSZPCNC. Prognostic value of tumor-infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303-1310.
- NE Thomas KBLF. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment, and melanoma study. J Clin Oncol. 2013;31:4252-4259.
- Cutaneous M: Melanoma: Cutaneous Melanoma. V.2.2022. NCCN Clinical Practice Guidelines in Oncology. Published online 2022. Accessed March 8, 2022. https://www.nccn.org/patientresources/patient-resources.
- Jiménez-Reinoso A, Nehme-Álvarez D, Domínguez-Alonso C, Álvarez- lina L. Synthetic TILs: Engineered Tumor-Infiltrating Lymphocytes With Improved Therapeutic Potential. Frontiers in Oncology. 2021;10:3454. doi:10.3389/FONC.2020.593848/BIBTEX.
- Hawkins RE, Jiang Y, Lorigan PC, et al. Abstract LB150: Clinical Feasibility and Treatment Outcomes with Unselected Autologous Tumor Infiltrating Lymphocyte Therapy in Patients with Advanced Cutaneous Melanoma. Published online July 1, 2021:LB150-LB150. doi:10.1158/1538-7445.AM2021- LB150.
- Sarnaik AA, Hamid O, Khushalani NI, et al. Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2021;39(24):2656-2666. doi:10.1200/JCO.21.00612.
What Centering the Patient Means: A Pharmacy Student Perspective
Arielle Davidson, PharmD Candidate
University of Michigan College of Pharmacy
MOQC Pharmacy Intern
Ann Arbor, MI
Emily Mackler, PharmD, BCOP
Michigan Oncology Quality Consortium
Michigan Institute for Care Management and Transformation
POEM Director
Ann Arbor, MI
While patients and caregivers have the most personal and informative experiences when it comes to health care, they are not always included in the care team. The Michigan Oncology Quality Consortium (MOQC) has found it imperative to center the patient and caregiver voice in everything it does and has created an entire council dedicated to their work, called the Patient and Caregiver Oncology Quality Council (POQC).
I am currently finishing my third year of pharmacy school and will be starting fourth-year rotations in the coming months. I am the Pharmacy Intern for MOQC and am involved with many different initiatives. Early on in my internship, MOQC leaders suggested that I should become involved with POQC and I feel so fortunate to have been able to work with this council for the last two years.
The council was started in 2017 with six members and has expanded to 16 members in its current state. These patients and caregivers take part in various workgroups that were started by members as passion projects, with immense support from MOQC. The current workgroups consist of financial navigation, caregiver and patient resources, Black Voices in Gynecologic Cancer: Understanding Experiences (BVOGUE), and recruitment. The financial navigation workgroup was started to address the overwhelming financial concerns associated with cancer. The caregiver and patient resources workgroup was created in an effort to prioritize caregivers and their needs, along with those of patients. BVOGUE is a project led by a POQC member that works to reduce racial disparities in the care of Black patients with gynecologic cancer. Lastly, the recruitment workgroup was established recently to increase the number of POQC members and promote diversity, equity, and inclusion within the council.
In addition to the dedicated POQC workgroups, members are also incorporated into every other initiative that MOQC has. For example, POQC members have helped to provide feedback on patient-reported outcome methods, various resources, oral chemotherapy program logistics, and much more. They also take part in the Steering Committee, which directs MOQC programs and efforts, as well as the Measures Committee, which chooses what MOQC will collect data on the following term. Full POQC meetings occur every six weeks, with workgroups typically meeting every other week. Members are invited to attend all MOQC regional and biannual meetings, with the opportunity to speak at any meeting.
While POQC is goal-oriented and accomplishes numerous tasks, the defining feature of this council is the culture that has been created. While working with this council the last two years, I can honestly say that this group has become more of a family to me. We are always there for each other, whether it be professionally or personally. Although 100% of our interactions are over Zoom, I still feel connected to all members. Oftentimes if I am having a bad day, joining a Zoom meeting with POQC will immediately make me feel better. Everyone on this team is so positive, understanding, and welcoming. They have accepted me with open arms and frequently show their appreciation.
It is very rewarding to be part of a group that has a shared goal and passion for improving patient care. With that kind of culture, our work doesn’t necessarily feel like work. One of the many things I love about POQC is being part of a community and being able to witness members relate to each other and form bonds about shared experiences. It is truly a unique companionship that brings many benefits, such as a great place to make connections as well as a support system to make any idea come to life.
As a future pharmacist, I have learned countless lessons from POQC that I will take with me into practice. The passion, drive, and positivity that everyone exudes daily really inspires me to display the same attitudes in my personal and professional life. They motivate me to always put the patient and caregiver first. Oftentimes, clinicians get very busy with hectic days and don’t prioritize the patient and caregiver or provide proper empathy for those interacting with this overwhelming diagnosis. When I am a pharmacist, I will always remind myself to take a step back and think of the patient and caregiver.
Another lesson that has become apparent throughout my time with POQC is that even if a patient or caregiver appears to “have it all together”, it doesn’t mean they do. They often feel as though they must be positive for themselves but also everyone else around them. I was specifically taught this lesson by one of the POQC members who confided in me to tell me that even though she always seems happy and outgoing, it is not always the case. Patients especially might not be completely honest with their providers because they do not want to feel like a burden. This has signaled to me that it is important to create a safe space for patients and caregivers by communicating that it is not a burden to hear how they are genuinely doing, and it will benefit their care if they are honest. In addition to the patient, I have learned how important it is to also focus efforts on the physical and mental health of the caregiver as they are just as much a part of the health care team, and often are relied on to absorb all the information being provided.
Since this council consists of patients and caregivers, they often have doctor appointments or don’t feel well and can’t make it to meetings. Our group is very understanding of this, flexible with meeting times, and emphasizes that family and health come first. This team makes it very clear that it is okay to miss any meeting for absolutely any reason. It is often said that if you are supposed to speak at a MOQC meeting and wake up that morning feeling nervous or unwell, it is completely acceptable to decline and there are no questions asked. This has taught me to demonstrate flexibility and accommodation with my coworkers.
I cannot emphasize enough how important it is to include patients and caregivers in quality improvement and care in general. The input of patients is what gives the work of MOQC meaning and purpose. Many of our initiatives would not be successful without the help that POQC members provide. They often help us to make sure that our patient-facing materials are patient-friendly, which is a huge task. They come up with ideas, problems, and initiatives that we didn’t know existed. They often share their own personal experiences with their journey that help us greatly.
For example, the financial navigation workgroup initially began their work by adding resources on our website. As their work progressed, some thought it would be more useful to educate physicians on financial burdens. This guided the creation of a breakout session in our most recent biannual meeting. We would have missed out on so many amazing opportunities, improvements, perspectives, and outcomes if we didn’t have the involvement of POQC.
As a future pharmacist, I am so lucky to have learned so many lessons from POQC that I will take with me into my career. I have learned to always put the patient’s feelings first, to truly ask patients how they are doing, focus on the caregiver, and to be as flexible as possible. While I have learned so much from POQC, the most important lesson is that patients and their caregivers absolutely need to be included in health care decisions, with no exceptions. They help to give our work perspective and make improvements that would not be possible without them. I am very hopeful for the future of POQC, as I know it will continue to grow and make a huge difference in the future of cancer care.
Characterization and Impact of Pharmacy Student Participation on Hematology/Oncology Advanced Pharmacy Practice Experiences
Matthew J Yacobucci, PharmD, BCOP
Assistant Professor, Albany College of Pharmacy and Health Sciences
Albany Medical Center Hospital
Albany, NY
Christina L Lombardi, PharmD
Cancer Care Service Line & Research Clinical Pharmacist
St. Peter’s Health Partners
Albany, NY
Laurie L Briceland, PharmD, FASHP, FCCP
Professor
Albany College of Pharmacy and Health Sciences
Albany, NY
Introduction
Oncology pharmacists practice in varied settings including inpatient hospitals, ambulatory care clinics, infusion centers, specialty pharmacies, and investigational drug services.1,2 Within these settings, many oncology pharmacists offer an Advanced Pharmacy Practice Experience (APPE), providing student pharmacists the opportunity to participate in collaborative team-based patient care, patient education, and clinical guideline development.
Literature characterizing the extent of participation and added value of APPE students to the oncology practice site, as well as the impact of the learning experience on the student pharmacist, is scarce. Two studies reported positive contributions of student pharmacists in assisting preceptors with oncology medication reconciliation or medication history-taking services.3,4 A survey of pharmacy practice department chairs reported that students on oncology rotations performed patient care work-ups, drug information requests, journal club presentations, and patient chemotherapy counseling.5
The value of student pharmacists’ contributions to patient care outcomes in diverse settings has been documented, though is lacking in the oncology setting.6-8 Documenting the full scope of activities and the associated value of the student to the practice site would provide useful information. Characterizing the professionalization impact of the student’s participation on oncology APPEs would provide equally useful information, as student pharmacists may enter the APPE lacking confidence in their abilities in the challenging oncology setting, and/or espousing preconceived notions that the rotation will be emotionally depressing and expose the student to dangerous chemicals.9,10 One study reported that student pharmacist learners in an oncology setting, upon participation in patient care services, gained a self-awareness of their purpose, and began to view themselves as a valuable health care team member, which furthered their professional identity formation.11
The purpose of our study of student pharmacist participation in hematology/oncology (hem-onc) APPEs was to characterize the scope of participation and impact of this participation upon the practice site, and upon student professionalization.
Methods
All students who completed a 6-week, hematology/oncology APPE, defined as inpatient or ambulatory care hem-onc, malignant hematology, or pediatric hematology, during 2016-2019 were retrospectively identified. For each hem-onc APPE, specific information from the student’s final APPE evaluation was extracted, including student-reported rotation activities, and 300 to 500-word self-reflections describing the meaningful impact of the APPE on the student. Rotation activities were grouped into like categorizations under two overarching classifications: Direct Patient Care and Education, or Guidelines, Policies, Standards, and Advocacy.12
To assess the impact of student contributions on the practice site, an electronic survey employing a 5-point Likert scale was developed and disseminated to the 33 preceptors of hem-onc APPEs. Preceptors ranked five activities from a list in which students had most impact and provided specific examples of what the site gained from having a student pharmacist. Preceptors also rated the level of entrustability typically placed upon student pharmacists who engaged in various activities at the practice site. The levels of entrustment range from observing only (Level I) to supervising junior colleagues (Level V), and at a minimum, literature suggests pharmacy students should graduate with the ability to complete activities with reactive supervision (Level III).13-15 APPE grades were reviewed from the preceptor final evaluations to serve as evidence of student aptitude in providing pharmacy services.
To determine the professionalization impact of the hem-onc APPE, each student’s APPE self-reflection was reviewed and no more than three themes of impact were extracted and categorized under one of the following groupings:16
Professionalization: career development; community service; leadership development; professionalization [confidence-building; self-directed learning; motivation; preceptor role model]; mentored others; self-awareness/habits of mind, including empathy, striving for accuracy; clarity/precision; and continuous learning);
Acquiring new/honing practice skills: immersion into new field of study; practice skills development; research; Patient counseling/communication skills: patient interactions; communication skills; teach/educate others; Collaborative teamwork: team-based pharmacy care; interprofessional education.
Results
Over the 3-year study period, 171 students completed hem-onc APPEs at private or hospital-affiliated ambulatory care settings (133; 77.8%) and/or inpatient (38; 22.2%) hospitals. All but seven students (>99%) earned a grade of ≥ B+, with no students failing a rotation.
The student collective self-reported 932 distinct participatory activities (5.5 per student), with the most common categories being: evaluating patient pharmacotherapy (209), providing in-service education to medical staff (132), providing patient counseling (non-chemotherapy) (99), answering drug information questions (96), and providing patient counseling (chemotherapy) (82). Most activities (89%) involved direct patient care and education.
Preceptor surveys were completed by 16 preceptors (48.5% response rate). Activities that preceptors entrusted students to perform with reactive supervision (level III) were medication reconciliation (46.7%) and in-service educational presentations to medical staff (53.3%). Activities that required direct supervision and specific instruction (level I) were chemotherapy patient counseling (37.5%) and research endeavors (33.3%). Three student activities that were most impactful to the site were: evaluating pharmacotherapy, providing medication education/adherence resources, and providing in-service educational presentations. The most common activities that would have occurred less frequently or not at all without students were in-service educational presentations to medical staff (50%), answering DI questions (41.7%), and research endeavors (41.7%).
There were 392 reflection themes of impact (2.3 per student) extracted and categorized from student self-reflections, distributed among the four overarching domains of professionalization (39.3%), which included self-awareness, developing empathy, confidence building/self-directed learning, and career development/ learning about oncology pharmacists’ roles; patient counselling/ communication skills (27.8%), which included patient interaction/ patient care activity; development of practice skills (20%), including learning a new field of study; and collaborative teamwork (13%), which included interprofessional education/team-based collaboration.
Discussion and Key Take-Aways
The majority of hem-onc APPEs were provided in the ambulatory care setting (77.8%). During their hem-onc APPEs, student pharmacists were involved in a broad range of direct patient care and educational activities, including evaluating and recommending pharmacotherapy, providing in-service education to medical staff, counseling patients, and researching drug information queries; our comprehensive compilation of participatory activities augments that reported in the literature.5 While this scope of student self-reported duties is not surprising, given that it mirrors much of the scope of practice of the oncology pharmacist,2,17 it was important to note that student pharmacists were given authentic direct patient care and education experience, comprising 89% of APPE activity. Not only is direct patient interaction an expectation of contemporary pharmacy APPE education,18 the provision of authentic patient care experiences is one underpinning of the development of professional identity formation in student pharmacists.19
Surveyed preceptors identified that the most impactful student activities included evaluating patient-specific pharmacotherapy, providing medication education/adherence resources directly to patients, such as filling pill boxes and compiling calendars, and educating healthcare colleagues by providing in-service presentations, which also was listed by 50% of preceptors as the most frequent activity to occur explicitly because of the presence of the student pharmacist. In addition, 42% of preceptors indicated that student pharmacists were instrumental in answering drug information queries and participating in clinical research, which would not have happened (or would have occurred less frequently) without the students’ presence.
These data indicate that preceptors found value in students’ day-to-day participation in patient care and education in the oncology setting and noted that some of these activities would occur less frequently (if at all) had it not been for the students’ presence. Further, preceptors entrusted students to perform medication reconciliation (46.7%) and in-service educational presentations to medical staff (53.3%) with reactive supervision (level III), which is the expected level of expertise of a pharmacy graduate.14,15
Others have also noted that students are well-positioned to provide medication reconciliation services, even in their early stages of clinical education, with appropriate supervision.3,17 Not surprisingly, activities that required direct supervision and specific instruction (level I) were chemotherapy patient counseling (37.5%) and research endeavors (33.3%), both of which would be considered patient-facing higher stakes activities in which the Hematology/Oncology Pharmacy Association recommends that pharmacists performing such activities be oncology-board certified (or gained the equivalent through practice).1,2 This information gathered from our oncology preceptor survey supports the contention that student pharmacists on hem-onc APPEs were entrusted contributors to the healthcare system.
Participation in hem-onc APPEs proved to be extremely impactful upon the professional identity formation of student pharmacists, as evidenced by student self-reflections on domains of professionalization, practice skills development, counselling/ communications skills, and collaborative teamwork. Professionalization was most prevalently extracted from student reflections, with a number focusing on self-directed lifelong learning opportunities, a critical strategy that students need to hone, especially in the dynamic cancer care environment.20
A number of students chose empathy as their theme of impact, noting that the frequent patient interactions/counseling provided great opportunity to develop empathy; students also expressed that the rotations were not melancholic, but rather uplifting and inspirational. Through our own experiences and those reported by Weingart,21 we have found many oncology patients to be upbeat and generally willing to openly communicate with the pharmacy team, owing to the high-stakes illness and treatments, longitudinal nature of the illness and frequent visits to the team, and involvement of family/support network. In sum, all themes of impact identified by student self-reflections meaningfully contributed to the student’s professional identity formation.
REFERENCES
- Ignoffo R, Knapp K, Barnett M, et al. Board-Certified Oncology Pharmacists: Their potential contribution to reducing a shortfall in oncology patient visits. J Oncol Pract. 2016 Apr;12(4):e359-68.
- Hematology/Oncology Pharmacy Association. Further defining the scope of hematology/ oncology pharmacy practice (2019). https://www.hoparx.org/images/hopa/resource-library/guidelines-standards/HOPA18_Scope-2_Web2.pdf (accessed 2020 Jun 24).
- Ashjian E, Salamin LB, Eschenburg K, et al. Evaluation of outpatient medication reconciliation involving student pharmacists at a comprehensive cancer center. J Am Pharm Assoc. 2015; 55(5):540-5.
- Phan H, Williams M, McElroy K, et al. Implementation of a student pharmacist-driven medication history service for ambulatory oncology patients in a large academic medical center. J Oncol Pharm Prac. 2019; 25(6):1419-1424.
- Kwon J, Ledvina D, Newton M, et al. Oncology pharmacy education and training in the United States Schools of Pharmacy. Curr Pharm Teach Learn. 2015;7:451-457.
- Whalen K, Aistrope DS, Ausili J, et al. The report of the 2016-17 Professional Affairs Standing Committee: Formally embracing and engaging preceptors in the Academy – the time has come. Am J Pharm Educ. 2017;81(9):Article S16.
- Mead T, Pilla D. Assessment of clinical and educational interventions that Advanced Pharmacy Practice Experience (APPE) students contributed to a family medicine residency program. Curr Pharm Teach Learn. 2017;9(3):460-467.
- Liu CH. Improving the quality of patient experience through student engagement. Am J Health-Syst Pharm. 2018(75):93-95.
- May P, Ladd J. Florida pharmacy students’ perspectives on careers in oncology. J Hematol Oncol Pharm. 2017;7(2):69-75.
- Ledbetter E, Lau S, Enterline A, et al. A simulation activity to assess student pharmacists’ knowledge and perceptions of oncology pharmacy. Am J Pharm Educ. 2020;84(5);Article 7474.
- Bates JS, Buie LW, Lyons K, et al. A study of layered learning in oncology. Am J Pharm Educ. 2016;80(4):Article 68.
- Holle LM, Boehnke Michaud L. Oncology pharmacists in health care delivery: vital members of the cancer care team. J Oncol Pract. 2014:(10):e142-e145.
- Haines ST, Pittenger AL, Stolte SK, et al. Core entrustable activities for new pharmacy graduates. Am J Pharm Educ. 2017;81(1):Article S2.
- Pittenger AL, Copeland DA, Lacroix MM, et al. Report of the 2016-2017 Academic Affairs Standing Committee: Entrustable professional activities implementation roadmap. Am J Pharm Educ. 2017;81:Article S4.
- Boyce EG, Harris CS, Bingham AL, et al. American College of Clinical Pharmacy Position Statement: Striving for excellence in experiential education. J Am Coll Clin Pharm. 2020;3(3):678-691.
- Briceland LL, Caimano CR, Rosa SW, Jablanski C, Veselov M. Post-APPE structured reflections: characterization of impactful learning themes and evidence of actionable professional development plans. 121st Annual Meeting American Association of Colleges of Pharmacy (Virtual 2020). Am J Pharm Educ: 2020:84(6): ajpe8220; DOI: https://www.ajpe.org/content/84/6/ajpe8220.
- Holle LM, Harris CS, Chan A, et al. Pharmacists’ roles in oncology pharmacy services: results of a global survey. J Oncol Pharm Pract. 2017;23(3):185-194.
- Rathbun RC, Hester EK, Arnold LM, et al. American College of Clinical Pharmacy. Importance of direct patient care in Advanced Pharmacy Practice Experiences. Pharmacother. 2012:32(4):e88-e97.
- Welch BE, Arif SA, Bloom TJ, et al. Report of the 2019-20 AACP Student Affairs Standing Committee. Am J Pharm Educ 2020; accepted draft ajpe8198; DOI: https://doi.org/10.5688/ajpe8198 (Accessed 2020 Jun 29).
- Thompson SA, Padilla ME, Loya AM, et al. Identifying opportunities for PharmD curricular reform by surveying oncology pharmacists about career preparedness and exposure. Curr Pharm Teach Learn. 2019;11:1205-12.
- Weingart SN, Cleary A, Seger A, et al. Medication reconciliation in ambulatory oncology. Jt Comm J Qual Patient Saf. 2007;33:750-757.
POLARIX - Has Polatuzumab Vedotin Finally Given R-CHOP the Axe in Upfront DLBCL?
Brian Bazzell, PharmD
Clinical Pharmacy Specialist - Hematology/Oncology
Ann Arbor Veterans Affairs Healthcare System
Ann Arbor, MI
For nearly two decades, treatment with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has been the standard approach for initial treatment of patients with diffuse large B-cell lymphoma (DLBCL). R-CHOP attains durable remissions in approximately 60% of patients, with those remaining in remission at 24 months attaining survival on par with the rest of the general population.1,2 However, 40% of those treated with R-CHOP will either have primary refractory disease or disease relapse within 24 months of initial treatment; many of these patients belong to high-risk subgroups known to confer a poor prognosis, including patients with genetic rearrangements in MYC and BCL2/BCL6 (often referred to as “double-hit” DLBCL).3
A number of clinical trials have attempted to improve survival outcomes in upfront treatment for high-risk patients, either via intensifying therapy (e.g. DA-R-EPOCH) or by adding novel drugs (e.g. lenalidomide, ibrutinib) in combination with R-CHOP.4–6 Unfortunately, all efforts to date have failed to improve survival outcomes compared to R-CHOP in first-line treatment of high-risk DLBCL, leading some to wonder if any novel treatment would succeed in this setting.
In December 2021, it appeared that the streak of negative trials in upfront DLBCL had finally come to an end. Results from the phase 3 POLARIX trial (polatuzumab + R-CHP vs R-CHOP) were first publicly presented at the American Society of Hematology (ASH) Annual Meeting and Exposition, with simultaneous online release of the manuscript in the New England Journal of Medicine.7 For the first time, a novel drug seemed to have improved outcomes compared to R-CHOP in upfront treatment of DLBCL, although controversy exists with regard to the impact these results may have on clinical practice. While some feel the results of POLARIX are practice-changing, others are less enthusiastic about the improvement, particularly given the high difference in cost between the regimens when vincristine is replaced with polatuzumab.
Given the controversy, POLARIX is a perfect trial to assess through the lens of oncology stewardship to determine whether polatuzumab-R-CHP should supplant R-CHOP as the treatment of choice in first-line treatment of DLBCL.
Polatuzumab vedotin in DLBCL
Polatuzumab vedotin is an antibody-drug conjugate that delivers the potent chemotherapeutic payload monomethyl auristatin E (MMAE) to DLBCL cells expressing CD79b, a component of the B-cell receptor, which is expressed on >90% of malignant B cells. Polatuzumab vedotin was initially granted accelerated FDA approval in June 2019 based on results of a single-arm, phase 2 trial in the relapsed/refractory DLBCL setting, in combination with bendamustine and rituximab in patients who had failed at least two prior lines of therapy.8 Rather than mandate a confirmatory phase 3 study in the relapsed/refractory DLBCL setting to attain full approval, the FDA opted to allow the drug’s manufacturer (Genentech/Roche) to use the POLARIX trial as its confirmatory phase 3 trial in the DLBCL space. This was allowed after publication of a promising phase 1/2b study of polatuzumab, rituximab, cyclophosphamide, doxorubicin, and prednisone (pola-R-CHP) in the upfront setting demonstrated an impressive overall response rate (ORR) of 89%, with 77% of those who responded attaining a complete response (CR).9
While allowing a phase 3 confirmatory trial to be performed in a different treatment setting is unusual for a drug with accelerated approval, it ultimately is beyond the scope of this feature. Also of note, vincristine was removed from the CHOP portion of the pola-R-CHP regimen due to concern for overlapping neurotoxicity with polatuzumab’s MMAE payload, as it also is a potent microtubule inhibitor.
POLARIX
The POLARIX trial is a phase 3, double-blind, placebo-controlled trial in which 879 de novo DLBCL patients were randomized 1:1 to receive either pola-R-CHP (n = 440) or R-CHOP (n = 439).7 Eligible patients were adults aged 18-80 years (median = 65 vs 66, with ~70% >60 years in both arms), treatment naive, with CD20+ disease, with an ECOG performance status of 0-2 (~85% = 0-1 in both arms, 15% = 2), and an International Prognostic Index of 2-5 (i.e. intermediate/high risk disease; IPI = 2 in 38% if both arms, IPI = 3-5 in 62% of both arms). Patients with double-hit DLBCL were eligible for the trial (7.9% vs 5.7%). Exclusion criteria included patients with a history of indolent lymphoma (i.e. transformation to DLBCL), previous receipt of an anthracycline, or CNS disease on diagnosis. The primary outcome was progression-free survival (PFS), while event-free survival (EFS, event = progression/relapse, death, biopsy confirmed residual disease after treatment completion, or initiation of new anti-lymphoma therapy), CR rate assessed via PET-CT, and overall survival (OS) were secondary outcomes. Patients in both the pola-R-CHP and R-CHOP treatment arms received 8 cycles of treatment; the first six cycles received were pola-R-CHP or R-CHOP, with two additional cycles of rituximab mandated per protocol. These two extra cycles of rituximab are not standard practice outside of the clinical trial setting, slightly limiting the real-world applicability of the trial and possibly inflating survival outcomes of these trial patients compared to real-world patients. Additionally, all patients were required to receive G-CSF prophylaxis during the first six cycles of treatment, which is not always done for patients receiving R-CHOP in clinical practice and could have lowered neutropenia rates compared to real-world cohorts.
At a median follow-up of 28.2 months, two-year PFS was significantly higher in the pola-R-CHP arm compared to R-CHOP (76.7% vs 70.2%, HR = 0.73, p = 0.02), with an absolute difference at 2 years in PFS between the two arms of 6.5%. Two-year OS (88.7% vs 88.6%, HR = 0.94, p = 0.75), ORR (85.5% vs 83.8%), and CR rate (78.0% vs 74.0%) were not significantly different between pola-R-CHP and R-CHOP. No significant differences were noted between the treatment arms with regard to safety outcomes; the most common adverse events were peripheral neuropathy (52.9% [1.6% grade ≥3] vs 53.9% [1.1% grade ≥3]), nausea (41.6% [1.1% grade ≥3] vs 36.8% [0.5% grade ≥3 ]), neutropenia (30.8% [28.3% grade ≥3] vs 32.6% [30.8% grade ≥3]), and diarrhea (30.8% [3.9% grade ≥3 ] vs 20.1% [1.8% grade ≥3]). Febrile neutropenia (14.3% vs 8.0%) was higher in the pola-R-CHP arm compared to R-CHOP despite primary G-CSF prophylaxis, although the rate of grade ≥3 infection was similar between the arms (15.2% vs 12.6%).
Oncology Stewardship Assessment
To fully assess the capacity of polatuzumab vedotin (and the POLARIX trial) to change clinical practice, it is imperative to assess the drug using oncology stewardship principles that factor in efficacy, safety, and value before reaching a final decision. Based on the data from POLARIX, pola-R-CHP was found to improve PFS by an absolute difference of 6.5% compared to R-CHOP, with no differences in ORR, CR rate, or OS and a similar toxicity profile (albeit with the addition of growth factor prophylaxis, which is an additional costly intervention). To fully assess the impact of a PFS difference of 6.5%, determining the number needed to treat (NNT) and expected cost of therapy for each regimen is essential. Based on the absolute risk reduction of 6.5% for two-year PFS found in POLARIX, the NNT of pola-R-CHP is calculated to be 15.4; in other words, 16 patients would need to receive pola-R-CHP to prevent one relapse compared to R-CHOP. While at face value this doesn’t sound unreasonable, the financial impact must be assessed to determine if this return is worth the cost required to attain it.
Cost of therapy can be determined using the wholesale acquisition cost (WAC) obtained from the Micromedex RedBook, which is a conservative and generalizable drug pricing benchmark that does not factor in rebates or discounts negotiated by individual health systems or retailers. Using WAC pricing and average patient characteristics (BSA = 2.0 m2, weight = 75 kg), the cost of six cycles of pola-R-CHP is approximately $152,185; using the same parameters treatment with six cycles of R-CHOP costs approximately $58,650, for a total difference in cost of ~$100,000 per patient treated (without factoring in the cost of G-CSF prophylaxis mandated by the trial).10 Given that 16 patients would need to be treated with pola-R-CHP in order to prevent one relapse with R-CHOP, we find that the total cost to prevent one DLBCL patient from progressing is approximately $1.6 million. Although salvage therapy options for relapsed/refractory DLBCL (e.g. high dose chemotherapy + autologous transplant, chimeric antigen receptor T-cell therapy) are also very expensive, pola-R-CHP does not currently appear to be a cost effective way to prevent subsequent progression or relapse, given the small impact it has on PFS in the upfront treatment setting and the significant cost of substituting it for vincristine at current price points.
Conclusion
In conclusion, although POLARIX did find an improvement in PFS compared to R-CHOP, which some may argue infers a higher fraction of patients who attained cure, the lack of an OS difference, small absolute difference in PFS, and massive increase in cost of therapy when using pola-R-CHP make it hard to recommend this treatment regimen over R-CHOP in frontline treatment of DLBCL at this time.
REFERENCES
- Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood, The Journal of the American Society of Hematology. 2010;116(12):2040-2045.
- Maurer MJ, Ghesquières H, Jais JP, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32(10):1066-1073.
- Liu Y, Barta SK. Diffuse large B‐cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94(5):604-616.
- Bartlett NL, Wilson WH, Jung SH, et al. Dose-adjusted EPOCH-R compared with R-CHOP as frontline therapy for diffuse large B-cell lymphoma: Clinical outcomes of the phase III intergroup trial alliance/ CALGB 50303. J Clin Oncol. 2019;37(21):1790-1799.
- Vitolo U, Witzig TE, Gascoyne RD, et al. ROBUST: First report of phase III randomized study of lenalidomide/R-CHOP (R2-CHOP) vs placebo/R-CHOP in previously untreated ABC-type diffuse large B-cell lymphoma. Hematol Oncol. 2019;37:36-37.
- Younes A, Sehn LH, Johnson P, et al. Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2019;37(15):1285-1295.
- Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N Engl J Med. 2022;386(4):351- 363.
- Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2020;38(2):155-165.
- Tilly H, Morschhauser F, Bartlett NL, et al. Polatuzumab vedotin in combination with immunochemotherapy in patients with previously untreated diffuse large B-cell lymphoma: an open-label, non-randomised, phase 1b–2 study. The Lancet Oncology. 2019;20(7):998-1010. doi:10.1016/ s1470-2045(19)30091-9.
- IBM Micromedex RED BOOK - Overview. Accessed April 15, 2020. https://www.ibm.com/products/micromedex-red-book.
