HOPA Publications Committee
Christan Thomas, PharmD, BCOP, Editor
Lisa Cordes, PharmD, BCOP, BCACP, Associate Editor
Renee McAlister, PharmD, BCOP, Associate Editor
Lydia Benitez, PharmD
Alexandra Della Pia
Jeff Engle, PharmD, MS
Karen M. Fancher, BS, PharmD, BCOP
Chung-Shien Lee, PharmD, BCOP, BCPS
Robert Steven Mancini, BCOP, FHOPA
Bernard L. Marini, PharmD
Alan L. Myers, PharmD, PhD
Gregory T. Sneed, PharmD
Diana Tamer, PharmD, BCOP
Kristin Held Wheatley, BCOP, PharmD
View PDF of HOPA News, Vol. 18, no. 4
Board Update: Four Councils, Immeasurable Impact
Larry Buie, PharmD, BCOP, FASHP
HOPA President (2021–2022)
Manager, Clinical Pharmacy Practice
PGY2 Residency Program Director, Memorial Sloan Kettering Cancer Center
New York, NY
Fall has set in, the leaves are changing, another American Pharmacist Month has passed, and I am half-way through my year as HOPA President. Although I am privileged to serve in this role, advocating for our members and our patients, I am by no means doing it alone. I work with a dedicated group of board members and I want to share important updates that are timely, impactful, and moving our organization forward.
Advocacy Council – Emily Mackler, Chair
On October 21, 2021, a total of 26 volunteers participated in a virtual HOPA Hill Day. The passion for our profession and patient care was on full display through a total of 43 meetings conducted with representatives from more than 20 states. In addition to educating members of Congress about the role of the hem/onc pharmacist, we asked for support for two important bills: Provider Status and Oral Chemo Parity. We couldn’t have done this without the leadership of the Public Policy Committee, Mark Hamm (Chair) and Jeffrey Pilz (Vice-Chair).
Also thanks to the work of the Advocacy Council, the Patient Advisory Panel now stands ready to provide patient perspectives, insights, and feedback to help inform the work of committees and task forces. You can find an article about them starting on page 22, of this issue of HOPA News.
Education Council – LeAnne Kennedy, Chair
HOPA’s virtual fall program, Emerging Trends + Models in Practice Management, was held on October 7, 2021 and attracted 295 registrants. Ninety-four percent of attendees said they were satisfied with the topics and 96% said they would recommend the program. We would like to thank Nicholas Baker (Chair) and Corbin Bennett (Vice-Chair) for their leadership in planning two independent tracks, Specialty Pharmacy and Investigational Drugs.
Planning is underway for HOPA’s Annual Conference 2022 (AC22), The Heart & Science of Cancer Care, which will take place in Boston March 30-April 2. Thirty-six sessions and 40 CEs are being planned. We are looking forward to seeing many of you in person for the first time in two years! HOPA will offer up to 60 travel grants to AC22 in Boston!
The last live education event scheduled for 2021 was BCOP Live!, a one-day virtual BCOP and ACPE learning experience held on November 30, 2021.
Professional Practice Council – David DeRemer, Chair
The Student Engagement Task Force, a time-limited, problem-solving initiative is winding down. But first, they will assemble the HOPA National Student Group (NSG) Committee, which will be run entirely by students, with the guidance of full pharmacist members. The NSG is charged with making the student voice heard and to empower students to decide how HOPA can most benefit them in terms of professional development.
Patient education is an important part of what we do. HOPA and our collaborators (NCODA, ONS, and ACCC) have launched and maintained two important chemotherapy patient education initiatives: IVCancerEdSheets.com and OralChemoEdSheets.com. Finally, the Council is leading a HOPA position statement on Chemotherapy Stewardship and the important role that oncology pharmacists play.
Research Council – Patrick Medina, Chair
At the time of this writing, an Oncology Landscape Survey was in the field and an Oncology Staffing Survey was just released by the Practice Outcomes and Professional Benchmarking Committee (POPBC). We look forward to sharing the insights gained from the survey results.
HOPA continues to offer research opportunities for pharmacists and new grants are available via the HOPA Research Fund and Early-Career Research awards. For those interested in developing additional research skills, HOPA is partnering again with ACCP for the FIT/MeRIT training programs. No matter how much or how little experience you have with clinical research, HOPA is here to support you.
Thank You, HOPA Volunteers!
The councils listed above support all four HOPA strategic imperatives and encompass more than 30 committees and task forces. We thank you all for everything that you do for HOPA and your patients. It would be impossible to accomplish any of our goals without your continued engagement.
I hope that you enjoy this edition of HOPA News – be sure to check out the articles on quality initiatives, credentialing, clinical pearls, member research, and much more. Finally, I hope that you all enjoy a wonderful holiday season. See you in 2022!
Feature: The FDA Accelerated Approval Program: A Double-Edged Sword
Jiyeon Joy Park, PharmD, BCOP
Clinical Assistant Professor - Rutgers University Ernest Mario School of Pharmacy
Clinical Pharmacy Specialist, Oncology - Rutgers Cancer Institute of New Jersey
Piscataway and New Brunswick, NJ
Introduction
In 1992, the US Food and Drug Administration (FDA) established the FDA Accelerated Approval Program in response to the HIV/ AIDS epidemic. Since then, the Program has allowed for faster approval of drugs for serious conditions that fill an unmet medical need especially in the field of oncology.1, 2 Under the Program, drugs are approved based on surrogate endpoints, which could be laboratory measurements, radiographic images, physical signs, or other measures that are thought to predict clinical benefit, but are not a direct measure of clinical benefit.
In 2012, the FDA Safety and Innovation Act was passed, which required drugs approved through the accelerated approval pathway to have surrogate endpoints that are reasonably likely to predict clinical benefit. Furthermore, the drugs approved through the Program need to demonstrate clinical benefit through confirmatory trials. If the confirmatory trial demonstrates clinical benefit, the FDA grants traditional approval for the drug. Otherwise, the FDA may withdraw the approval.1 While the Program makes for a speedy approval process that could be as short as a few months, there has been a significant number of application and indication withdrawals of targeted agents and immune checkpoint inhibitors in the recent months due to the failure to demonstrate benefit in confirmatory trials.2, 3
Accelerated Approvals and Withdrawals of Drugs/ Biologics in Oncology
According to Center for Drug Evaluation and Research (CDER) as of June 30, 2021, there were a total of 269 accelerated approvals for both oncology and non-oncology indications since the birth of the Program. More than 60% of these approvals were for oncologic indications, and the majority occurred just in the last 10 years. Most of the studies that led to accelerated approvals in oncology used either objective response rate (ORR) or progression-free survival (PFS) as a surrogate endpoint. Out of more than 180 oncology approvals, less than half of the approvals were successfully converted to full FDA approvals so far.3 To remind healthcare professionals, the package labeling of the approved products has a statement such as: “This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).”
One noteworthy example of a drug withdrawn after drawing attention for its initial promising results is olaratumab (Lartuvo). Olaratumab was originally approved for soft tissue sarcoma (STS) in 2016 based on the PFS benefit in a phase 1b/2 trial. It was widely expected to change the horizon of STS treatment by introducing a first-in-class targeted therapy for STS. However, olaratumab failed to demonstrate overall survival (OS) benefit in the confirmatory phase 3 trial (ANNOUNCE trial).4, 5 As a result, olaratumab was voluntarily withdrawn by the manufacturer (Eli Lilly) in 2020.3, 5
The FDA has also requested manufacturers to withdraw their products if they failed to conduct confirmatory trials. For instance, the manufacturer (Sanofi-Aventis) for fludarabine phosphate (Oforta), never conducted the confirmatory trial due to lack of commercial demand and difficulty with subject recruitment. Subsequently, the FDA asked the manufacturer to voluntarily withdraw the product.3, 6
Accelerated Approval and Withdrawal of Immune Checkpoint Inhibitors
Since the advent of the Program, the FDA has granted accelerated approvals to various targeted agents, including many of the immune checkpoint inhibitors. According to CDER as of June 30, 2021, there were more than 50 approvals involving 8 immune checkpoint inhibitors—ipilimumab, nivolumab, pembrolizumab, durvalumab, atezolizumab, avelumab, cemiplimab, and dostarlimab. However, just in the last two years, there have been at least 7 withdrawals of indications involving immune checkpoint inhibitors, which is the greatest number of withdrawals at any given time since the beginning of the Program.3 Table 1 provides an overview of withdrawn oncology drugs/biologics from January 2020 to September 2021.
Nivolumab for Small Cell Lung Cancer
Nivolumab (Opdivo) was granted accelerated approval in 2018 for the treatment of patients with small cell lung cancer (SCLC) whose disease had progressed after platinum-therapy and at least one other line of therapy. The approval was based on the phase 1/2 CheckMate-032 trial studying nivolumab versus nivolumab plus ipilimumab in patients with advanced or metastatic solid tumors.7 In the SCLC cohort, an objective response was observed in 10% of patients in the nivolumab 3 mg/kg group, 23% in the nivolumab 1 mg/kg plus ipilimumab 3 mg/kg group, 19% in the nivolumab 3 mg/kg plus ipilimumab 1 mg/kg group, and 33% in the nivolumab 1 mg/kg plus ipilimumab 1 mg/kg group.7 In CheckMate-331, one of the confirmatory studies, there was no statistically significant difference in OS between patients who received nivolumab versus those who received topotecan or ambirubicin. The median OS was 7.5 versus 8.4 months respectively (HR 0.86; 95% CI 0.72-1.04; P=0.11).8 The other confirmatory study (CheckMate-451) also did not show OS benefit in nivolumab plus ipilimumab versus placebo (median OS 9.2 vs. 9.6 months, HR 0.92; 95% CI 0.75-1.12; P=0.37).9 As a result, the nivolumab indication for SCLC was withdrawn in December 2020.10
Nivolumab for Hepatocellular Carcinoma
Nivolumab was initially approved in 2017 for treatment of patients with hepatocellular carcinoma who were previously treated with sorafenib. Recently, the manufacturer (Bristol Myers Squibb) announced that nivolumab would be voluntarily withdrawn after failing to meet post-marketing requirements in the confirmatory trial.11 The accelerated approval was based on tumor response rates in CheckMate-040, a multicenter, non-comparative, open-label phase 1/2 study. In the dose expansion phase of the study, the objective response was seen in 42 out of 214 patients (20%; 95% CI 15-26) receiving nivolumab 3 mg/kg IV every 2 weeks. Three patients had complete responses and 39 had partial responses.12 The confirmatory trial (CheckMate-459) in 2019 did not meet the primary endpoint of OS. The median OS was 16.4 months for nivolumab and 14.7 months for sorafenib (HR 0.85; 95% CI 0.72-1.02; P=0.0752) which was not statistically significant.13
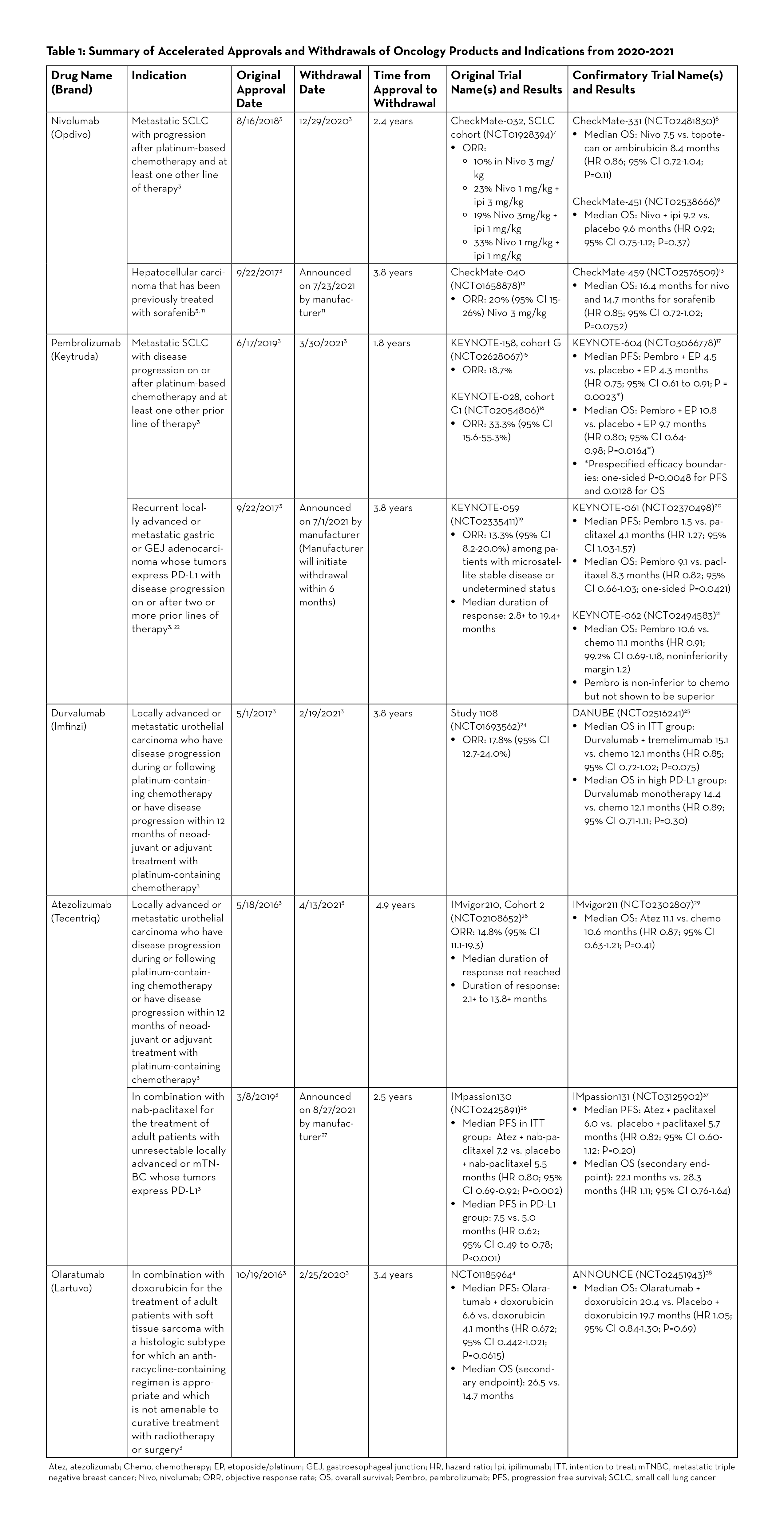
Pembrolizumab for Small Cell Lung Cancer
The results from KEYNOTE-158 (cohort G) and KEYNOTE-028 (cohort C1) studies led to the accelerated approval of pembrolizumab (Keytruda) in 2019 for metastatic small cell lung cancer with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy.14 These two trials reported ORR of 18.7 and 33.3%, respectively.15, 16 KEYNOTE-604, the confirmatory trial, reported mixed results for the dual primary endpoints of PFS and OS. While the study met prespecified efficacy boundary for PFS, it did not meet the efficacy boundary for OS. Median PFS was 4.5 versus 4.3 months for pembrolizumab plus etoposide/ platinum (EP) and placebo plus EP, respectively (HR 0.75; 95% CI 0.61-0.91; P=0.0023), and median OS was 10.8 versus 9.7 months (HR 0.80; 95% CI 0.64-0.98; P=0.0164). The prespecified efficacy boundaries were one-sided P = 0.0048 for PFS and 0.0128 for OS.17 In March 2021, Merck, the manufacturer for pembrolizumab, announced voluntary withdrawal for the indication.18
Pembrolizumab for PD-L1-Positive Gastric or Gastroesophageal Junction Adenocarcinoma
Pembrolizumab gained accelerated approval in 2017 for the treatment of PD-L1-positive recurrent locally advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma after two or more lines of therapy.3 The approval was based on KEYNOTE-059 study, an open-label, multicenter, non-comparative, multi-cohort trial, which demonstrated ORR of 13.3% (95% CI 8.2-20).19 In one of the confirmatory trials (KEYNOTE-061), pembrolizumab did not significantly prolong OS (HR 0.82; 95% CI 0.66-1.03; one-sided P=0.0421). Median OS was 9.1 months for pembrolizumab versus 8.3 months for paclitaxel. Median PFS was 1.5 months and 4.1 months, respectively (HR 1.27; 95% CI 1.03-1.57).20 In another confirmatory trial (KEYNOTE-062), pembrolizumab was studied as monotherapy and in combination with chemotherapy. While pembrolizumab was shown to be non-inferior to chemotherapy, it was not superior to chemotherapy. Median OS with pembrolizumab was 10.6 months versus 11.1 months with chemotherapy (HR 0.91; 99.2% CI 0.69-1.18, noninferiority margin 1.2).21 Merck, the manufacturer for pembrolizumab, announced on July 1, 2021 that it will withdraw pembrolizumab for this specific indication. Currently, pembrolizumab is still approved in combination with trastuzumab, fluoropyrimidine- and platinum-containing chemotherapy for patients with HER2-positive gastric or GEJ adenocarcinoma.22
Durvalumab for Locally Advanced or Metastatic Urothelial Carcinoma
Durvalumab (Imfinzi) was granted accelerated approval in May 2017 for previously treated locally advanced or metastatic urothelial carcinoma (mUC), and the indication was voluntarily withdrawn by its manufacturer, AstraZeneca, in February 2021.23 The initial approval was based on Study 1108, a phase 1/2 trial which reported an ORR of 17.8% (95% CI 12.7-24.0%) in patients with locally advanced or mUC.24 The confirmatory trial (DANUBE trial) analyzed coprimary endpoints of OS compared between durvalumab monotherapy and chemotherapy groups in the population with high PD-L1 expression and between durvalumab plus tremelimumab and chemotherapy groups in the intention-to-treat population. The results demonstrated no significant OS benefit with durvalumab in both the high PD-L1 and the intention-to-treat populations. The median OS in the durvalumab monotherapy versus chemotherapy in the high PD-L1 group was 14.4 versus 12.1 months (HR 0.89; 95% CI 0.71-1.11; P=0.30). The median OS in the intention-to-treat population was 15.1 in the durvalumab plus tremelimumab group versus 12.1 months in the chemotherapy group (HR 0.85; 95% CI 0.72-1.02; P=0.075).25
Atezolizumab for PD-L1 Positive Metastatic Triple Negative Breast Cancer
Atezolizumab (Tecentriq) was granted accelerated approval in March 2019 for the treatment of adult patients with unresectable locally advanced or metastatic triple negative breast cancer (mTNBC) whose tumors express PD-L1 in combination with protein-bound paclitaxel (nab-paclitaxel). The approval was based on the phase 3 IMpassion130 study which demonstrated favorable PFS. In patients with PD-L1-positive tumors, the atezolizumab plus nab-paclitaxel group had median PFS of 7.5 months versus 5.0 months in the placebo plus nab-paclitaxel group (HR 0.62; 95% CI 0.49 to 0.78; P<0.001).26 The subsequent confirmatory study (IMpassion131) did not meet the primary endpoint of PFS. Although the approval status was initially maintained after the FDA Oncology Drugs Advisory Committee (ODAC) convened in April 2021 and voted to keep atezolizumab, in August 2021, the manufacturer (Roche/Genentech) announced that it will voluntarily withdraw atezolizumab, stating that “…due to the recent changes in the treatment landscape, the FDA no longer considers it appropriate to maintain the accelerated approval.”27
Atezolizumab for Locally Advanced or Metastatic Urothelial Carcinoma
Atezolizumab received accelerated approval in May 2016 for the treatment of patients with locally advanced or mUC who have disease progression during or following platinum-based chemotherapy, or whose disease has worsened within 12 months of receiving neoadjuvant or adjuvant platinum-based chemotherapy.3 The approval was based on the results of IMvigor210 study, which demonstrated ORR of 14.8% (95% CI 11.1-19.3%) in locally advanced or mUC patients who received atezolizumab 1200 mg IV every 3 weeks. The median duration of response not reached during the study.28 In the confirmatory trial (IMvigor211) published in 2018, the median OS was not shown to be statistically significant between the atezolizumab group and the chemotherapy group in patients with mUC who had progressed after platinum-based chemotherapy.29 Subsequently, the FDA assigned another study as a confirmatory trial (IMvigor130); however, the manufacturer (Roche) decided to voluntarily withdraw atezolizumab as the second-line treatment of mUC.30 The data from IMvigor130 trial showed statistically significant PFS benefit in previously treated locally advanced/mUC patients; and while the interim OS data noted a positive trend, the full data analysis is pending.31 Atezolizumab still holds accelerated approval for the treatment of locally advanced or mUC patients who are not eligible to receive cisplatin-containing chemotherapy.32
Re-Approval after Withdrawal
Sometimes, withdrawn products have come back on the market after a modification of indications and/or warnings. For instance, gefitinib (Iressa) was on the market for 10 years since its accelerated approval for the treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC), before it was withdrawn for its failure to demonstrate OS benefit. However, two years later, the FDA approved gefitinib for a more specific indication for the treatment of epidermal growth factor receptor mutation-positive NSCLC.33 In another example, gemtuzumab ozogamicin (Mylotarg) was approved for the treatment of relapsed CD33-positive acute myeloid leukemia (AML) in patients 60 years or older, but was withdrawn due to its failure to confirm clinical benefits and safety concerns including early mortality and veno-occlusive disease. It was re-approved years later at a lower dose for a different patient population (newly diagnosed CD33 positive AML patients).3, 34
Tracking Accelerated Approvals and Product Withdrawals
As many new drugs and biologics become approved, it may be challenging to keep track of the approval or withdrawal status of a product or an indication. The FDA publishes a summary report on drug and biologic accelerated approvals.2 In addition, the FDA ODAC conducts meetings to evaluate and review data concerning the safety and effectiveness of oncology drugs and make appropriate recommendations to the Commissioner of the FDA.35 Decisions on whether to maintain or withdraw accelerated approval of oncology drugs and biologics are made during the ODAC meetings, which are open to the public via webcast.36 The FDA also provides an email alert service (https://www.fda.gov/about-fda/contact-fda/get-email-updates) which sends out updates that can be tailored to oncology drugs and market withdrawals.
Conclusion
Surrogate endpoints do not always translate to clinical benefits or prolonged survival as observed in some of the oncology drugs/biologics approved through the FDA Accelerated Approval Program. Also, there seems to be highly variable amounts of time between accelerated approvals and the completion of confirmatory trials. The majority of withdrawals from the Program have so far involved oncology products, and immune checkpoint inhibitors have especially accounted for most of the withdrawals—perhaps due to a large number of accelerated approvals compared to other drugs and biologics. As much as the FDA Accelerated Approval Program brings in new therapy options and indications to the market with increased speed, healthcare professionals including pharmacists should also be vigilant of any approval status changes or withdrawals.
REFERENCES
- US Food & Drug Administration. FDA approves pembrolizumab for metastatic small cell lung cancer. Updated June 18, 2019. Accessed September 24, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-metastatic-small-cell-lung-cancer
- Chung HC, Lopez-Martin JA, Kao SCH, et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J Clin Oncol. 2018;36(15)doi:10.1200/JCO.2018.36.15_suppl.8506
- Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients With extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2017;35(34):3823-3829. doi:10.1200/JCO.2017.72.5069
- Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369-2379. doi:10.1200/JCO.20.00793
- Merck. Merck provides update on KEYTRUDA® (pembrolizumab) indication in metastatic small cell lung cancer in the US. Updated March 1, 2021. Accessed September 24, 2021. https://www.merck.com/news/merck-provides-update-on-keytruda-pembrolizumab-indication-in-metastatic-small-cell-lung-cancer-in-the-us/
- Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi:10.1001/jamaoncol.2018.0013
- Shitara K, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123-133. doi:10.1016/S0140-6736(18)31257-1
- Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571-1580. doi:10.1001/jamaoncol.2020.3370
- Merck. Merck provides update on KEYTRUDA® (pembrolizumab) indication in third-line gastric cancer in the US. Updated July 1, 2021. Accessed September 25, 2021. https://www.merck.com/news/merck-provides-update-on-keytruda-pembrolizumab-indication-in-third-line-gastric-cancer-in-the-us/
- AstraZeneca. Voluntary withdrawal of Imfinzi indication in advanced bladder cancer in the US. Updated February 22, 2021. Accessed September 29, 2021. https://www.astrazeneca.com/media-centre/press-releases/2021/voluntary-withdrawal-imfinzi-us-bladder-indication.html
- Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411. doi:10.1001/jamaoncol.2017.2411
- Powles T, van der Heijden MS, Castellano D, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1574-1588. doi:10.1016/S1470- 2045(20)30541-6
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108- 2121. doi:10.1056/NEJMoa1809615
- Roche. Roche provides update on Tecentriq US indication for PD-L1- positive, metastatic triple-negative breast cancer. Updated August 27, 2021. Accessed September 24, 2021. https://www.roche.com/media/releases/med-cor-2021-08-27.htm
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-20. doi:10.1016/S0140-6736(16)00561-4
- Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748-757. doi:10.1016/S0140-6736(17)33297-X
- Roche. Roche provides update on Tecentriq US indication in prior-platinum treated metastatic bladder cancer. Updated March 8, 2021. Accessed September 24, 2021. https://www.roche.com/media/releases/med-cor-2021-03-08.htm
- Galsky MD, Arija JAA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10236):1547-1557. doi:10.1016/S0140-6736(20)30230-0
- Tecentriq (atezolizumab) [package insert]. South San Francisco, CA: Genentech Inc; 2021.
- Center for Drug Evaluation and Research. Application number: 206995Orig1s000 summary review. Updated July 13, 2015. Accessed September 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206995Orig1s000SumR.pdf
- US Food & Drug Administration. FDA Approves gemtuzumab ozogamicin for CD33-positive AML. Updated September 1, 2017. Accessed September 24, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-gemtuzumab-ozogamicin-cd33-positive-aml
- US Food & Drug Administration. Oncologic drugs advisory committee. Updated February 18, 2021. Accessed September 30, 2021. https://www.fda.gov/advisory-committees/human-drug-advisory-committees/oncologic-drugs-advisory-committee
- US Food & Drug Administration. October 28, 2021: meeting of the oncologic drugs advisory committee meeting announcement. Updated September 3, 2021. Accessed September 30, 2021.
- Miles D, Gligorov J, Andre F, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/ metastatic triple-negative breast cancer. Ann Oncol. 2021;32(8):994-1004. doi:10.1016/j.annonc.2021.05.801
- Tap WD, Wagner AJ, Schoffski P, et al. Effect of doxorubicin plus olaratumab vs doxorubicin plus placebo on survival in patients with advanced soft tissue sarcomas: the ANNOUNCE randomized clinical trial. JAMA. 2020;323(13):1266-1276. doi:10.1001/jama.2020.1707
Thanatology for Oncology Pharmacists
Robert Mancini, PharmD, BCOP, FHOPA
BMT Pharmacy Program Coordinator & PGY2 Oncology Pharmacy Residency Director
St. Luke’s Cancer Institute, Adjunct Clinical Instructor – Oncology
Idaho State University College of Pharmacy
Boise, ID
No, this is not an overview of the most recent Avengers movies, but rather a discussion on a concept we all face every day as oncology practitioners, death and dying. While my first thought when learning the term was the Titan Thanos, the concept is not far off. The term thanatology is derived from the Greek mythology “Thanatos,” which is the personification of death. Thanatology as a professional discipline came into the public limelight in the 1960s and 70s after two key publications, The Meaning of Death by Herman Fiefel in 1959 and On Death and Dying by Elisabeth Kubler-Ross in 1969.1-2 In the latter, Kubler-Ross outlined the now-famous five stages of grief: denial, anger, bargaining, depression, and acceptance.
We as pharmacists, especially those practicing in oncology, deal with death and dying on a frequent basis. The most recent statistics from the American Cancer Society state that the 5-year overall survival rate for all cancer combined is approximately 67%, indicating a mortality rate of 33%. Said another way, one in three patients will die from their cancer.3 This may differ depending on the type and stage of cancer, and is unmatched in other areas of practice outside hospice.3 Despite this, pharmacist education/ training on topics surrounding death are woefully inadequate.
Surveys suggest 68% of pharmacy schools include some form of death education in their curriculum, compared to 95-96% of nursing and medical schools. Most of us would likely state we are not prepared to have conversations with patients about the topic.4 In fact, in a study evaluating pharmacist perspectives after implementation of physician-assisted suicide, 93% of respondents said their pharmacy degree did not prepare them for said interactions.5 While some schools have started to focus more education with guided electives, there is a lot more we can do to help pharmacists in their education related to death and dying.4,6
In looking back at my journey with this topic, in my teaching at Idaho State University (ISU) College of Pharmacy, I can reach as far back as my first year of Pharmacy School. In my Law and Ethics course during my P1 year, I was asked to read Tuesdays with Morrie by Mitch Albom. That was the first impact that made me realize there was more to being a pharmacist than the therapeutics. During my fourth year APPEs, by which time I had already realized I wanted to specialize in oncology, I was able to spend one of my rotations at an inpatient hospice facility working with an interdisciplinary team whose whole focus was care of the dying patient.
Then, during my PGY2 in oncology, there was a conversation that caught me off guard. Though it was more than a decade ago, I still remember it. I was dispensing lenalidomide to a gentleman in his 80’s who asked me: “If this were you, would you do it?” How does one answer that question when it isn’t me; when my life has not been as long or full as his? So many thoughts ran through my mind, most notably, why was I never prepared to have this conversation?
Fast forward a few years, I am now the Residency Program Director (RPD) for our PGY2 program and I am finding more and more oncology pharmacy residents struggling with the same questions. This led me to two points, first to develop an elective for pharmacy students to think and talk more openly about this topic, but second to ensure that I incorporated talks related to this during hospice and palliative care rotations for my PGY2 residents. Since then, I have found a huge benefit for students, residents, and myself to continue to review and focus on this topic.
Now, the question becomes, how can you integrate such topics in your own practice, either for yourself or for your students, residents, employees, or coworkers? Former students, residents and colleagues have introduced me to many of these resources over the years, and those introductions have proven invaluable.
For me personally, one of the books that helped me most in my approach to patient care was Mitch Albom’s The Time Keeper.7 It centers around the concept of the time we have on this earth with two competing paradigms, a young girl who is ready to give up on life and an old man who wants to live forever. It really helped me realize that there is no one patient specific factor that can predict how a person will react to the decision to pursue treatment for cancer. By looking beyond what we see and understanding the patient’s goals and priorities, I was able to learn how to better discuss such difficult conversations, even when I may not have agreed with them personally.
While the articles and guidelines tell us what we should do in someone with Stage IV breast cancer, for example, they can never address the personal decision making that goes into those choices. This has helped me better accept the choices our patients make, including the reality of death and acceptance of that inevitable endpoint. I believe this has been a key component in avoiding emotional burnout in my practice.
There are a multitude of resources depending on how in depth you want to go. Reading many of these books and reviewing these resources (see table 1) have helped me in many ways over the years including finding the need to bring more of this education to our pharmacy students and residents. For those in academia, it is important to find ways to incorporate this topic into your classes or electives, wherever possible. A survey of students at ISU who took my elective course found that their self-assessment in “awareness of the complex psychosocial issues that oncology patients face,” increased by over 5 points (on a scale of 1-10). There are many ways I have incorporated this theme into my teaching opportunities including both scientific and theoretical resources. I actually require my elective students to choose one of those books to read for the course and write a paper putting themselves in the shoes of a terminal cancer patient to understand why someone may or may not pursue treatment.
All in all, there are myriad opportunities to expand our education and acceptance of death and dying if we are willing to breach the topic and take it head on. As a profession we can do more to help guide our patients through these struggles once we accept the reality of our situation. This could also potentially help reduce emotional burnout once we make this type of education more common place in pharmacy education.
REFERENCES
- Fiefel, H (Ed.). (1959). The meaning of death. McGraw-Hill.
- Kubler-Ross, E. (1970). On death and dying. Collier Books/Macmillan Publishing Co.
- Siegel RL, et al. Cancer Statistics 2021. CA Cancer J Clin. 2021;71:7-33.
- Beall JW, Broeseker AE. Pharmacy Students’ Attitudes Toward Death and End-of-Life Care. Am J Pharm Educ. 2010;74(6): 104
- Parr RB, et al. The Pharmacist and patient death: Are we prepared for the emotional and professional impacts? Res Social Adm Pharm. 2019;15: e20
- Manolakis ML, et al. A Module on Death and Dying to Develop Empathy in Student Pharmacists. Am J Pharm Educ. 2010;75(4): Article 71
- Albom, M (2012). The Time Keeper. New York: Hachette Books
Credentialing and the Role of the Hematology/Oncology Clinical Pharmacy Specialist within the US Department of Veterans Affairs
Andrea (Annie) Bailey, PharmD, BCOP
Clinical Pharmacy Specialist – Hematology/Oncology
Phoenix VA Health Care System
Phoenix, AZ
Julia M. Hammond, PharmD, BCOP
Clinical Pharmacy Specialist – Hematology/Oncology
Durham VA Health Care System
Durham, NC
The Veterans Health Administration (VA) is the country’s largest integrated health care system with 9 million veterans receiving care each year. One of the core values of the VA is to strive for the highest quality of health care.1 Clinical Pharmacy Specialists (CPS) play an integral role in providing high quality care to veterans including in the hematology/oncology practice setting. Hematology/oncology CPSs practice at the height of their license through comprehensive medication management (CMM) services. The hematology/oncology CPS team works autonomously and alongside the care team to provide direct patient care and improve clinical outcomes.
Scope of Practice and Ongoing Professional Practice Evaluation
The Clinical Pharmacy Practice Office (CPPO) was created in 2010 within the Pharmacy Benefit Management (PBM) service to advance and support the role of the CPS. CPPO’s role is to optimize clinical pharmacy across the VA, provide cost-effective care, and improve both patient access and care. In the VA, CPSs operate under a scope of practice, or credentialing, which is like a collaborative practice agreement that is used outside the VA. However, the scope of practice is between the CPS and the individual VA facility and does not reside with an individual physician. With a scope of practice, CPSs have the authority to modify, initiate, or discontinue medications, order labs and imaging, and manage medication toxicities.2,3
The credentialing process to obtain an initial scope of practice requires the pharmacist meet competency criteria as determined by the Executive Committee Medical staff (ECMS) or Professional Standards Board (PSB) and chief of pharmacy. This may include an individualized mentorship training in which the pharmacist seeking scope of practice approval is assigned a pharmacy specialist mentor. The mentorship period includes observation of a minimum number of patient care cases and chart review. The assigned mentor provides recommendations for the appropriateness for scope of practice approval.
After ECMS or PSB review of the individual pharmacist’s competencies and approval for credentialing process, critical duties outlined in the scope must be assessed. This includes a Focused Professional Practice Evaluation (FPPE) in which competencies are assessed relating to the duties and functions that are outlined in the scope of practice. The FPPE process allows the pharmacist to function autonomously to demonstrate knowledge, skill, and competence required for the requested scope of practice. The results of the FPPE are provided to ECMS for final approval of the scope of practice.4
After approval of the initial scope of practice, CPSs are required to undergo Ongoing Professional Practice Evaluations (OPPE). This process includes peer reviews to ensure quality care and re-assess CPS knowledge and competence. The OPPE process is locally defined by each VA facility but should be performed at minimum, biannually. Results of OPPE are utilized during re-credentialing and renewal of the CPS scope of practice, which occurs approximately every 2 years.4,5
As of February 2021, there were over 130 hematology/oncology CPSs within the VA. Hematology/oncology CPSs are highly trained with many completing both general PGY1 and PGY2 oncology residency training. In addition, many have obtained Board Certification in Oncology Pharmacy (BCOP). Hematology/oncology CPSs provide care in both the inpatient and outpatient settings providing expertise in antineoplastic medication selection, medication monitoring, preparation/dispensing, antineoplastic toxicity management, patient education, and cost savings considerations.6
Advanced Practice Providers within the VA
Within the VA, hematology/oncology CPSs are essential to the care team. Through utilization of the scope of practice, hematology/oncology CPSs serve as advanced practice providers. Hematology/oncology CPSs have prescriptive authority, allowing them to practice at the height of their license. The primary role of the hematology/ oncology CPS is CMM focusing on antineoplastic treatment. Their expertise focuses on treatment appropriateness and selection, safety, and patient/staff education. The CPS works closely with the care team to provide patient-centered care to the veteran including collaboration with the hematology/oncology provider for management beyond the CPS scope of practice.
Furthermore, hematology/oncology CPSs have an important role in supportive care considerations. The CPS ensures appropriate anti-emetics are ordered to prevent chemotherapy induced nausea and vomiting. This includes being involved in the process of ensuring appropriate order sets and utilizing their scope of practice to order anti-emetics, as necessary. In addition, many hematology/ oncology CPSs within the VA utilize their scope to order appropriate agents for the prevention of cancer-related infections, prevention of hypersensitivity reactions, and other supportive care agents.
By ordering appropriate lab tests and providing patient monitoring, hematology/oncology CPSs play a key role in identifying anti-neoplastic toxicities including immune-mediated adverse effects and anti-neoplastic adverse effects. CPSs are able to identify toxicity early in treatment which can help prevent more severe toxicity as well as assisting in anti-neoplastic dose adjustments and discontinuation. CPSs can utilize a scope of practice to manage adverse effects. Examples include refractory nausea/vomiting, diarrhea, constipation, dermatological toxicities, and myelosuppression.
In addition, hematology/oncology CPSs play an important role in education and training. The VA provides training opportunities for medical students and residents, hematology/oncology fellows, pharmacy residents and students. The hematology/oncology CPS provides educational services and assistance to trainees. Further, the hematology/oncology CPS is often responsible for providing in-service education to the care team regarding treatment updates, new drug information, and appropriate administration of antineoplastic agents.
Although the practice of the hematology/oncology CPS depends on the specific VA facility, hematology/oncology CPSs are important in the management, monitoring, and follow-up of oral antineoplastic agents. This can include utilization of telehealth services through an oral antineoplastic clinic. The hematology/ oncology CPSs use their scope of practice to order lab results to ensure appropriate monitoring. Further, prescriptive authority is used to renew oral antineoplastic orders and manage toxicities. Regular follow up between the patient and the hematology/oncology CPS allows for assessment of adherence and toxicity of oral antineoplastic medications and ensures appropriate monitoring per FDA labeling/Guideline recommendations.
Ultimately, hematology/oncology CPSs play an integral role in the care of hematology/oncology patients within the VA Health Care System. They are highly trained advanced practice practitioners who help guide the selection, management, and monitoring of antineoplastic agents. The care team relies on the CPS to provide patient and staff training as well as ensure appropriate supportive care efforts are achieved with hematology/oncology patients. The hematology/oncology CPS utilizes a scope of practice that allows them to function independently with prescriptive authority. The VA gives hematology/oncology CPSs the opportunity to practice at the height of their license and professional training to ensure positive outcomes in the care of hematology/oncology patients.
REFERENCES
- US Department of Veterans Affairs. About VA. Available at https://www.va.gov/about_va/Accessed October 16, 2021.
- Pharmacy Benefit Management. Pharmacy Benefits Management Services. Clinical Pharmacy Practice Office. Available at https://www.pbm.va.gov/PBM/CPPO/Clinical_Pharmacy_Practice_Office_Home.asp. Accessed October 16, 2021.
- McFarland, Groppi, Jorgenson, et al. 2020. Role of the US Veterans Health Administration Clinical Pharmacy Specialist Provider: Shaping the Future of Comprehensive Medication Management. Can J Hosp Pharm. 73(2): 152–158.
- VHA handbook 110811: Clinical pharmacy services. US Department of Veterans Affairs, Veterans Health Administration; 2017. [cited 2017 Dec 12]. Available at www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120. Accessed October 16, 2021.
- Pharmacy Benefit Management Guidance. Professional Practice Evaluations for VHA Pharmacists with a Scope of Practice; 2021. Accessed October 16, 2021.
- Clinical Pharmacy Specialist Role in Oncology Depart of Veterans Affairs Fact Sheet; 2021. Accessed October 16, 2021
A Pharmacist’s Perspective along the Quality Improvement Journey
Marie Anne Louis-Jeune, PharmD, BCPS
Pharmacy Safety & Quality Coordinator
Memorial Cancer Institute
Hollywood, Florida
Amy L. Morris, PharmD
Coach, ASCO Quality Training Program
Clinical Pharmacist, Leukemia/MDS
UVA Health
Charlottesville, VA
Please Provide Background about Yourself, your Team, and your Quality Improvement Project.
Dr. Louis-Jeune currently serves as the Pharmacy Safety and Quality Coordinator at Memorial Cancer Institute (MCI). Dr. Morris serves as faculty for the American Society of Clinical Oncology (ASCO) Quality Training Program (QTP), and coached this team on their Quality Improvement (QI) project. She is a Clinical Pharmacist at UVA Health.
During Fiscal Year 20220, MCI welcomed ~74,000 patient visits; increased volume was accompanied by treatment start delays. Due to cleanroom optimizations to meet USP 797 & 800 requirements, concern arose that pharmacy was potentially the rate limiting step in chemotherapy initiation. Our institutional initiative, therefore, was to improve patient wait times for chemotherapy, and our project focused specifically on decreasing time to chemotherapy initiation for patients admitted to the hospital.
Our core team consisted of two physicians, the Director of Pharmacy, and myself. Senior leadership buy-in and involvement was key. The VP of MCI at the time sponsored our teams’ training; he is an ASCO QTP faculty member. The MCI Director of Quality and a Clinical Pharmacist from UVA Health served as our team’s QTP coaches.
How was the Problem and Aim Statement Developed?
Although patient satisfaction scores indicated there were issues with “wait time,” our institution did not have benchmarks or detailed reports confirming and quantifying delays to initiating chemotherapy. We started this project assuming our focus would be on the outpatient infusion services. The team quickly learned we would be more successful selecting a small population and honing in on a specific issue, resulting in a shift of our focus to oncology patients treated in the inpatient setting, evaluating time to first chemotherapy. We could then adopt the findings and processes to a generalized population, i.e outpatient clinics.
We missed a major key factor that is necessary throughout any successful quality improvement project: Data! This was a lightbulb moment for me. This project would require our team to replace subjective theories with objective facts.
First, we needed to develop a problem statement which clearly defines what does not meet stakeholders’ needs. It must be objective (no blaming and shaming) and quantifiable, so our team had to gather data manually from the electronic medical record to quantify our actual wait times. We used the 5Ws – Who, What, Where, When, and Why to create our problem statement. Once we identified the average time to chemotherapy, we created a SMART (Specific, Measurable, Achievable, Relevant and Timely) aim statement to define the goal of our project. Originally we approximated a time reduction, however, utilizing externally published benchmark data helped to establish an achievable time reduction goal.
What were the Project’s Deliverables?
In addition to the problem statement, embedded in the aim was an overarching timeline that further guided time-specific targeted milestones. However, it was evident that each team member’s schedule varied on a day-to-day basis and patient care was top priority. Therefore, from the start of the project, we established a recurring meeting to meet our deliverables. The active involvement of our coaches kept the momentum during each meeting.
Our multidisciplinary team first mapped out the current state process, highlighting each operational step and decision point from patients’ arrival to the unit to time to first chemotherapy administration. Using sticky notes to jot down each process allowed us to rearrange the map easily as we worked. We had several learning opportunities during this process and decided to integrate additional frontline team members due to gaps in knowledge of inpatient workflows. We optimized our team through the involvement of the Advanced Practice Providers Supervisor and Nurse Manager of the inpatient oncology department.
What was the Process for Evaluating Data?
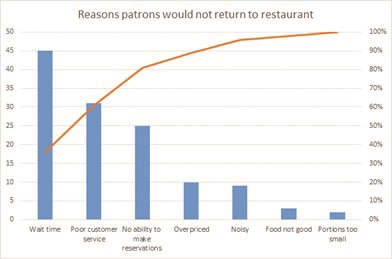
Our team used different QI tools to analyze data. We brainstormed potential causes of chemotherapy delay on a cause-and-effect diagram, also called a fishbone diagram. However, the tool that impacted our work the most was the Pareto chart, which truly told a story using the data! A Pareto chart prioritizes the most frequent causes and displays that 20% of causes contribute to 80% of the problem (see table 1).
The Pareto can be hard to imagine if you haven’t created one before, so below is a simple Pareto sample chart outlining the reasons for patrons not to return to a restaurant. If the restaurant owner did not gather data from his guests, but instead assumed his loss of revenue and empty restaurant was due to the chef and the quality of the food, he would be mistaken. Although this seems simplistic, often in healthcare the causes of system-wide problems are not fully analyzed and quantified, and efforts to fix the problem are less impactful if solutions do not focus on the highest contributors to the issue.
Our Pareto chart objectively highlighted and eliminated subjective assumptions about causes of delays. Data demonstrated pharmacy did not solely contribute to delays in chemotherapy administration. Quantifying causes for delay we recognized issues with lab collection and reporting were contributing the most to delays and set the groundwork for identifying process improvements that would make the most impact.
Improvements?
The next step was to implement our first intervention via a Plan, Do, Study, Act (PDSA) cycle. PDSA allows for planning and doing the intervention, but more importantly studying the outcomes and acting based on those measured outcomes. Some teams may meet their aim through the implementation of just one or two PDSA cycles, but it is important to remember that in QI, multiple PDSA cycles are usually needed to provide a robust outcome.
With the data provided from the Pareto chart and process map depicting the current workflow and all team members available, we brainstormed on different process and workflow optimizations that would aid in meeting our aim. To determine our first intervention, we utilized the priority matrix and assigned each recommendation to the appropriate quadrant depending on the amount of effort and ease of implementation. Implementing improvements that fall in the low effort and high impact quadrant lead the highest chance of success in meeting the aim statement. The first PDSA focused on the workflow for processing labs. Our physician team members served as the liaisons to the physician group; this step clearly demonstrated that physician group buy-in was successful through our team physician champion’s aide. We established a timeline to collect data that would provide meaningful information. At the end of PDSA #1, we recognized the benefit of the process improvement although we did not meet our aim. Therefore, we implemented PDSA #2 while continuing PDSA #1.
It was important for our team to understand that all QIs may not yield the expected outcomes or meet aim. However, we could always take what we learned to tailor future improvements. In conclusion, we reached our aim and determined sufficient data was available to move forward.
What was the Hardest Part of the Project?
I expected the hardest part of this project to reflect the beginning stages of the Kubler-Ross Change curve; as the project progresses from creating alignment, maximizing communication and sparking motivation, the team may face emotions such as shock, denial, frustration and depression. Very quickly, I realized that some things are out of our control. The COVID-19 pandemic threw a wrench in our timeline, and ultimately the entire QI project. Along with the nation, our department immediately focused its efforts to meet the national and organization’s guidelines to prevent the spread and provide relief to the shortage of healthcare workers. Our aim, timeline, and goals were altered. The number of admissions within our targeted patient population decreased. The momentum, that was once there, was shifted. Additional meetings with the coaches, including buy-in from leadership, were crucial to identify how to successfully regroup and refocus.
What Would you have done Differently?
I was so eager to make an impact and see this project through but didn’t realize QI does not end once data collection is complete. An effective QI requires continuous data evaluation to monitor shifts and trends. Through the manual data collection process, our team realized an important key member was missing; an information technology (IT) specialist, who should be involved in every QI. IT contribution may have saved time from all the manual data collection I performed throughout the improvement. In order to sustain the outcomes, I worked with IT to build a report providing the process time stamps identified. This learning point will contribute to future QI projects I lead or participate in.
What’s next?
Data, results, process optimizations and automation all play a role in the next key step in sustaining the improvement. Next, we will present the outcome and recommendations to the leadership team for buy-in and sponsorship to standardize the process throughout the oncology departments. Our team has taken the next steps to disseminate knowledge gained during the QTP project, and we will participate in and coach future QIs within our department.
For details of the QI project: https://meetings.asco.org/abstracts-presentations/201949
REFERENCE
- Vulfovich M, Salzberg M, Louis-Jeune M, et al. Decreasing inpatient chemotherapy initiation delays at Memorial Regional Hospital. J Clin Oncol. 2021:39 (suppl 28); abstr 225.
The Past, Present, and Future Front-Line Treatment Strategies for Chronic Lymphocytic Leukemia
Anthony J. Perissinotti, PharmD, BCOP
Clinical Pharmacist Specialist - Hematology
Michigan Medicine: University of Michigan
Ann Arbor, MI
Chronic lymphocytic leukemia (CLL) is the most prevalent type of leukemia in the United States. With a median age at diagnosis of 72, CLL primarily affects older adults; many of whom have several comorbidities.1, 2 As a result, treatment selection necessitates caution. As our understanding of the underlying biology improves, there has been a trend away from the use of cytotoxic chemotherapy and toward oral targeted therapies. This paradigm shift brings new challenges, which pharmacists must be aware of to stay ahead of the curve and lead change.
The Past
Chemotherapy was once the most common treatment modality for patients with CLL. Fludarabine, cyclophosphamide, and rituximab (FCR) or bendamustine and rituximab (BR) were prescribed to young, fit patients, whereas BR or chlorambucil with obinutuzumab (O-Clb) were given to older patients with comorbidities.3-6 Unfortunately, these treatments did not result in disease cure, and the prognosis for patients with high-risk cytogenetics were dismal.4 In fact, the only chance for cure was for patients to receive an allogeneic cell transplantation.7 One caveat is that FCR has shown durable remissions for patients with mutated IGHV.6, 8, 9 It can, however, only be recommended to a small set of CLL patients who are young, fit, and have mutated immunoglobulin heavy chain variable region gene (IGHV), as acute toxicities prohibit its use in older patients.10 Furthermore, even in young individuals, the risk of secondary malignancy makes this an unappealing alternative.3 In summation, new therapies were desperately needed.
The Present
A number of front-line therapy trials demonstrated the safety and efficacy of Bruton Tyrosine Kinase (BTK) inhibitors (ibrutinib and acalabrutinib) and venetoclax with obinutuzumab for first-line CLL compared to chemotherapy.11-16 These included the front-line ECOG 1912 (FCR versus ibrutinib with rituximab), Alliance A041702 (BR versus ibrutinib versus ibrutinib with rituximab), RESONATE-2 (chlorambucil versus ibrutinib), iLLUMINATE (O-Clb versus ibrutinib with obinutuzumab), ELEVATE-TN (O-Clb versus acalabrutinib versus acalabrutinib with obinutuzumab), and CLL14 (O-Clb versus venetoclax with obinutuzumab) trials.11-16 In the relapsed/refractory (R/R) setting, the RESONATE (ofatumumab versus ibrutinib), ASCEND (BR or idelalisib with rituximab versus acalabrutinib) and MURANO (BR versus venetoclax with rituximab) studies also paved the transition away from chemotherapy toward targeted therapies.17-21 Two major questions remain: first, which BTK inhibitor should be chosen, and second, should BTK inhibitors be selected instead of a venetoclax-based regimen?
With the success of targeted therapies such as ibrutinib came new challenges, including unique toxicity profiles. Despite being a BTK inhibitor, ibrutinib inhibits several kinases including TEC, EGFR, ITK, BMX, ERBB4, among others.22 This may lead to “off-target” side effects such as atrial fibrillation, hypertension, bleeding, diarrhea, dermatitis, and myalgias.23 Randomized controlled trials presented at this year’s American Society of Clinical Oncology Annual Meeting (ASCO 2021) and European Hematology Association Congress (EHA 2021) sought to determine whether second generation BTK inhibitors acalabrutinib or zanubrutinib are better tolerated than ibrutinib.16, 24 For patients with R/R CLL, ALPINE compared zanubrutinib to ibrutinib, whereas ELEVATE-RR evaluated acalabrutinib versus ibrutinib. Collectively, these data imply patients may tolerate second generation BTK inhibitors better than ibrutinib, but the “off-target” toxicities are not completely abrogated with the more selective second generation BTK inhibitors. For example, in ELEVATE-RR ibrutinib increased the rate of any grade atrial fibrillation (16% versus 9.4%; p=0.02), minor bleeding (51.3% versus 38%; p < 0.05), hypertension (23.2% versus 9.4%; p < 0.001), and interstitial lung disease or pneumonitis (6.5% versus 2.5%; p = 0.0241) compared to acalabrutinib. Additionally, diarrhea, arthralgia, back pain, muscle spasms, and dyspepsia occurred more frequently with ibrutinib, whereas headache and cough occurred more frequently with acalabrutinib.16 Similar results were found in ALPINE which demonstrated a lower rate of any grade atrial fibrillation/flutter with zanubrutinib compared to ibrutinib (10.1% vs 2.5%; p = not reported) while neutropenia was higher with zanubrutinib (28.4% versus 21.7%) but the increased risk for neutropenia did not appear to increase the risk for infections. There was no difference in hypertension or major hemorrhage but longer follow-up and/or larger studies are necessary to determine whether these and other adverse effects are truly different.24
One practical consideration clinicians should keep in mind when choosing a BTK inhibitor is that acalabrutinib and zanubrutinib were both evaluated on a twice-daily schedule, which may affect adherence rates. Nonadherence to BTK inhibitors has been associated with poor outcomes. Regardless of which BTK inhibitor is chosen, the therapy will be indefinite and will necessitate years of continuous follow-up, managing adverse events, navigating financial assistance, and assessing adherence by pharmacists and other members of the care team.
The question of whether a BTK inhibitor should be used instead of a venetoclax-based regimen will most likely not be answered until the results of the CLL-17 study (comparing ibrutinib vs. venetoclax/obinutuzumab vs. ibrutinib/venetoclax, in frontline CLL) are reported. In the absence of data demonstrating superior efficacy of BTK inhibitors over venetoclax, cost should be a major consideration. In the CLL-14 trial, patients received venetoclax for only one year, rather than the alternative approach of an indefinite BTK inhibitor.14 There is an initial expense and time commitment when treating patients with venetoclax, due to tumor lysis syndrome monitoring and simultaneous use of an intravenous anti-CD20 monoclonal antibody; however, data have shown overall costs are significantly reduced over time because patients can remain off therapy.25 In fact, long-term follow-up data from the CLL-14 trial have shown the majority of patients can remain off therapy for several years when treated with venetoclax/obinutuzumab despite only a year of therapy.14, 26 At four years, 81% of patients have still not required additional CLL therapy. Not only were remissions durable, they were also deep. Venetoclax/obinutuzumab led to high rates of undetectable measurable residual disease (uMRD) in peripheral blood in 42% of patients via Clonoseq Assay/next generation sequencing (<10-6) and 76% of patients via an allele-specific oligonucleotide PCR assay (<10-4) at the end of treatment.14 Patients who are unlikely to remain progression-free and off therapy for an extended period of time (e.g., patients with TP53 mutated CLL) may not benefit from this cost savings, thus the therapy choice may shift back to BTK inhibitors given the durable outcomes that have been reported in this population.27, 28 Furthermore, with the ongoing COVID-19 pandemic, BTK inhibitors are an appealing choice because they do not require frequent exposure to the healthcare system and do not necessitate the use of a B-cell directed monoclonal antibody, which has important implications for the efficacy of COVID-19 vaccines.29 Ultimately, both BTK inhibitors and venetoclax/obinutuzumab are exceptional new standards of care for CLL. Shared decision making with patients outlining the pros and cons of each treatment approach should drive therapy decisions.
The Future
Combining a BTK inhibitor with venetoclax is a promising future strategy. Deep remissions can be achieved, and like venetoclax/obinutuzumab, patients can come off therapy after a specified period of treatment. Several approaches are being used to investigate a variety of combinations.30-36 Double and triple combinations (incorporating anti-CD20 monoclonal antibody), MRD-directed therapy (using uMRD to indicate when to stop therapy or detectable MRD to guide when to continue therapy), and combinations with a finite duration irrespective of MRD are among these approaches. It is unlikely these combinations will improve overall survival for most patients with CLL over sequential therapy, and even if they do, it will take a decade to reveal this benefit. In the short term, our goal should focus on keeping patients off therapy for as long as feasible while also lowering the overall economic burden on patients and the healthcare system. Attaining deep remissions does not guarantee this. Time off therapy and overall costs need to be a prioritized outcome. In current trials, rates of MRD- are a major focus, however, not discontinuing therapy until MRD- and/or treating MRD relapse may inadvertently lead to additional time on therapy and healthcare costs without improvements in quality of life or overall survival. Since venetoclax/obinutuzumab is the current finite standard of care, it should be used as a benchmark for establishing if a BTK inhibitor in combination with venetoclax +/- obinutuzumab genuinely improves the aforementioned clinical endpoints. Combination therapy will be utilized in practice soon but whether these combinations improve quality of life, decrease the cost of care, decrease patients’ time off therapy, or improve overall survival will not be known for years to come.
REFERENCES
- National Cancer Institute. Accessed October 8, 2021. seer.cancer.gov/statfacts/html/clyl.html.
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. Jan 2021;71(1):7-33. doi:10.3322/caac.21654
- Kutsch N, Bahlo J, Robrecht S, et al. Long Term Follow-up Data and Health-Related Quality of Life in Frontline Therapy of Fit Patients Treated With FCR Versus BR (CLL10 Trial of the GCLLSG). Hemasphere. Feb 2020;4(1):e336. doi:10.1097/hs9.0000000000000336
- Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. Dec 28 2000;343(26):1910-6. doi:10.1056/nejm200012283432602
- Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. Mar 20 2014;370(12):1101-10. doi:10.1056/NEJMoa1313984
- Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. Jan 14 2016;127(2):208-15. doi:10.1182/ blood-2015-06-651125
- van Gelder M, de Wreede LC, Bornhäuser M, et al. Long-term survival of patients with CLL after allogeneic transplantation: a report from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. Mar 2017;52(3):372-380. doi:10.1038/bmt.2016.282
- Thompson PA, Tam CS, O’Brien SM, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. Jan 21 2016;127(3):303-9. doi:10.1182/blood-2015-09-667675
- Rossi D, Terzi-di-Bergamo L, De Paoli L, et al. Molecular prediction of durable remission after first-line fludarabine-cyclophosphamide-rituximab in chronic lymphocytic leukemia. Blood. Oct 15 2015;126(16):1921-4. doi:10.1182/ blood-2015-05-647925
- NCCN Clinical Practice Guidelines in Oncology. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, v1.2022.
- Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N Engl J Med. Dec 27 2018;379(26):2517-2528. doi:10.1056/NEJMoa1812836
- Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. Jan 2019;20(1):43-56. doi:10.1016/s1470- 2045(18)30788-5
From Resident to Director: Understanding the True Value of Residency
Sarah Hogue, PharmD
Director Oncology Pharmacy
St. Luke’s Health System
Boise, ID
I feel I have always been a leader and my story starts as a child. I was that typical kid that was told she was bossy, which now I like to think of as showing great leadership potential. I naturally gravitated towards leadership positions in high school and throughout college, serving in leadership roles in many student organizations. I completed a Pharmacy Administration Advanced Pharmacy Practice Experience during my fourth year of pharmacy school and this prompted me to seek out Administration rotations in both my post-graduate year residencies.
Residency Experience Solidified an Interest in Management
During my Post Graduate Year 2 (PGY2) Oncology Residency, I had a longitudinal Administration rotation that included presenting at the Oncology Pharmacy and Therapeutics subcommittee, attending various other meetings, and two concentrated weeks spent with the Director of Pharmacy. I know what you all are thinking, “ugh, meetings” and I will tell you that I felt that way at first as well, but then getting to see all the brilliant minds in one room, working together, and making decisions was exciting. I was able to see firsthand how integrated the Pharmacy Director was in all operations within the cancer center, not just those that directly involve pharmacy. From these experiences, I knew that eventually I would like to be in a leadership position wherever I ended up practicing.
I had interactions with the Director throughout the year as he was my direct supervisor. He had an open-door policy and was always friendly, inviting, and helpful. It was a nice dynamic to have my Residency Director to guide me through all things residency related, but still have a relationship with the Director to be a resource for life in general as well. During the residency year, he was not only my supervisor, but also a mentor.
Leadership, Inspiration, and Advocacy go Hand in Hand
During my PGY2 residency, my interest in management was solidified. The Pharmacy Director showed me how you can be a leader while simultaneously showing genuine compassion and interest in your team members. He was a source of inspiration, an advocate for pharmacy across the system, pushed his team to operate at the top of their license, supported any projects that the team brought forward, and encouraged growth and professional development. All of this was also true for the residency program. He made me feel like I was an integral member of the team, even as a resident.
Halfway through the residency year, I accepted a job offer for a position where I would be the primary pharmacist at a small community cancer center. My goal for this position was to develop pharmacy services and integrate pharmacy into the healthcare team. This was a daunting task and over the next six months I took every opportunity to work with the Director and glean as much information from his as possible about how to manage a practice.
Fast forward three years, and I am back at St. Luke’s where I completed my PGY2 residency working with that same Pharmacy Director. I found my way back to St. Luke’s, largely because of the ongoing relationship I had with the Director. During the next three years, I wore a couple of different hats and then the Director announced his retirement. He told me that he would like me to take over for him when he leaves. I am honored, humbled, and scared.
The Student Becomes the Director
Now, here I am as the new Oncology Pharmacy Director for St. Luke’s and I get to be in the role of the mentor and advocate for the residency program. We have three PGY2 residents each year and I am the primary preceptor for their longitudinal Administration rotation and their direct supervisor. I work closely with our PGY2 Residency Director to make sure the residents have a successful year. I absolutely love working with our residents and seeing how much they learn throughout the year and then take that out into practice. My relationship with them is similar to the relationship I had with my Pharmacy Director as a resident.
As the Pharmacy Director, I see myself as a resource for the residents and an advocate for the residency program. While the Residency Director manages many aspects of the residency program, I participate in interviews and am a member of the Residency Advisory Committee. I make sure that we have funds to send residents and preceptors to conferences where they can share their experiences with other pharmacy professionals across the nation and bring back valuable information to our organization. I also make sure that the residents have the resources they need, whether it is reference materials, office space, or IT equipment.
I also support resident projects by guiding the residents as to who the stakeholders are and helping them make those connections outside of our department. Additionally, I support the residents by ensuring that they are maintaining a healthy work-life balance, and have positive relationships with the pharmacy staff, their preceptors, and the Residency Director.
Residency Programs Drive Profession Forward
Residency Programs are an incredibly valuable asset to the organization. Having residents pushes our entire pharmacy team to be at the very top of their game. Residency projects help drive our profession and practice forward. Many of the pharmacy initiatives we have been able to implement over the years have been the direct result of residency projects, including a pharmacist-led oral chemotherapy service, the addition of a full-time Pediatric Oncology Pharmacist, and many pharmacy protocols to help with oncology patient management.
Working with the PGY2 residents is one of my favorite aspects of my job. I always learn something from them, and they make our organization and our pharmacy team stronger every year.
Feature: von Hippel-Lindau (VHL) Disease and the Newly FDA Approved Agent Belzutifan
Karin Abernathy, PharmD
PGY2 Oncology Pharmacy Resident
Vanderbilt University Medical Center
Nashville, TN
Von Hippel-Lindau (VHL) disease is a multisystem neoplastic predisposition disorder due to autosomal dominant mutations in the VHL tumor suppressor gene. Tumors may affect the central nervous system (CNS), kidneys, adrenal glands, pancreas, and reproductive organs.1 The most common manifestations associated with VHL disease include CNS hemangioblastomas, clear cell renal carcinoma (RCC), and pancreatic neuroendocrine tumors (pNETs). Benign, visceral cysts also occur frequently in the kidneys, pancreas, or epididymis (in males) and may be detected in combination with malignant VHL-associated tumors.
VHL disease is present in about 1 in 36,000 individuals and may present in childhood, adolescence, or adulthood, with a mean age at initial presentation of 26 years. Life expectancy for patients with VHL disease is low, between 40-52 years, and VHL-related mortality is most commonly due to complications of RCC and CNS tumors. The majority of affected individuals will have a positive family history through a germline mutation, but up to 20% of cases arise from de novo mutations. The development of VHL disease is most often related to Knudson’s two-hit hypothesis of hereditary tumorigenesis, in that patients inherit a germline mutation of the VHL gene from an affected parent and a wildtype gene from the unaffected parent. The germline mutation of the gene represents the first hit, and if a somatic mutation occurs and results and inactivates the normal allele (second hit), the individual is then prone to tumor formation.2
The Pathogenesis of VHL Disease
The VHL gene is located on chromosome 3p25 and encodes the VHL protein that functions as a tumor suppressor. Additionally, this VHL protein forms a multiprotein complex with elongin B, elongin C, and cullin 2, which collectively targets several proteins for proteasomal degradation, subsequently regulating their levels within the cell. The VHL component in the complex functions as an E3 ubiquitin ligase for target molecules that once covalently bound, undergo degradation by the proteasome. Other functions of the VHL protein include regulation of cytokinesis, control of microtubule function, and regulation of the cell cycle.1
Involved in the pathogenesis of VHL disease are hypoxia-inducible factor (HIF) 1 and 2, which are two of the major proteins regulated by VHL. In general, these transcription factors regulate glucose transport, lipid metabolism, pH homeostasis, and angiogenesis. The protein complex including VHL is additionally responsible for ubiquitin-mediated degradation of HIF. Therefore, in instances of loss of function of the VHL gene, there is sustained expression of pro-tumorigenic molecules that include vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), erythropoietin, and transforming growth factor alpha (TGF-α). Collectively, these upregulated target molecules lead to cell proliferation, angiogenesis, and tumorigenesis.2
The diagnosis of VHL disease is generally made by a positive family history, detection of a germline pathogenic VHL gene variant, and presence of at least one VHL-associated tumor. Specific pathogenic variants or deletions of the VHL gene can influence the phenotype or clinical manifestations of disease. Variable presentations of VHL disease have led to classification of VHL subtypes, type I and type II, based on the presence of pheochromocytoma (a type of neuroendocrine tumor found on the adrenal glands). Type I disease has a very low risk of pheochromocytomas (generally absent), with higher risk for retinal and CNS hemangioblastoma, RCC, pancreatic cysts, and other neuroendocrine tumors. Type II VHL is associated with high risk for pheochromocytomas and is further subcategorized by additional risk of RCC, with type 2A conferring low risk of RCC and type 2B conferring high risk of RCC. Type 2C VHL is a disease consisting only of pheochromocytoma. Deletions in VHL, as well as nonsense and truncating variants appear more commonly in type I disease, while missense variants are more common in type II disease.2
Risks, Diagnosis, and Complications of VHL
In order to prevent severe VHL-associated complications, the VHL Alliance has published suggested active surveillance guidelines in patients at risk for VHL disease, or those known to have VHL but do not yet have symptoms. At any age, patients with a family history may choose to undergo genetic testing and counseling to determine their risk for developing VHL disease and need for surveillance. This includes annual eye and neurologic examinations started at the age of 1 in pediatric patients known to carry the VHL mutation. By the time patients are ages 5 and older, biannual quality ultrasounds or MRI (preferred) should be completed to assess the areas of the kidneys, pancreas, adrenals, brain, and spine to assess for any abnormalities.3
CNS hemangioblastomas are the most common tumors in patients with VHL disease and tend to present with multiple lesions. Annual retinal examinations are crucial in vision preservation and early MRI screening is indicated in patients at risk for VHL disease to establish an earlier diagnosis and minimize disease related complication. The most common complication in patients who survive to older than age 60 is clear cell renal cell carcinoma, in approximately 70% of VHL patients.2,3
Effects of belzutifan for Adult Patients with VHL
In August of 2021, the FDA approved belzutifan (MK-6482) via priority review for adult patients with VHL requiring therapy for associated RCC, CNS hemangioblastomas, or pNETs, not requiring immediate surgery. Belzutifan is a small molecule inhibitor of hypoxia-inducible factor 2 alpha (HIF-2α), a transcription factor that regulates genes that promote adaptation to hypoxia. Under normal oxygenation conditions, HIF-2α is targeted for ubiquitin-proteasomal degradation by the VHL protein. In patients with VHL disease, the dysfunctional VHL protein results in the stabilization and accumulation of HIF-2α. When belzutifan binds to HIF-2α, this prevents the interaction between the HIF-2α and hypoxia-inducible factor 1 beta, resulting in reduced transcription and expression of target genes that are associated with cellular proliferation, angiogenesis, and tumor growth.4,5
The effects of belzutifan were investigated in the ongoing Study 004 (ClinicalTrials.gov identifier NCT034401788), a phase 2, open-label study comprised of 61 patients with VHL-associated RCC. This was diagnosed based on a VHL germline alteration and with at least one measurable solid tumor localized to the kidneys. The diagnosis of RCC could be radiologic evidence only (no histologic diagnosis required). Other patients enrolled in the trial had other VHL-associated tumors including CNS hemangioblastomas and pNETs. Participants in the study could not have received any prior treatment with HIF-2α inhibitors or other systemic anti-cancer therapies. Any patients with immediate need for surgical intervention or with evidence of metastatic disease were excluded from the trial.4
In this single arm study, patients received belzutifan 120 mg orally once daily until either disease progression or unacceptable toxicity. The primary endpoint was overall response rate by radiology assessment with additional endpoints including duration of response and time to response. In the study population, the median age was 41 years [range 19-66 years], 53% were male, 90% were white, and 82% had an ECOG performance status of 0. VHL Type 1 disease represented 84% of the patients and median time from diagnosis of VHL to enrollment was 17.9 months [range 2.8-96.7]. Prior surgical procedures for RCC had been performed in 77% of patients.4
Follow-up for the trial patients was a minimum of 12 months. The overall response rate (ORR) was 49% (95% CI: 36, 62) in the patients with VHL-RCC. The median duration of response in the VHL-RCC patients was not reached; 56% of responders had a duration of response of at least 12 months, and a median time to response of 8 months. In patients with other VHL-associated non- RCC tumors, there were 24 patients with measurable CNS hemangioblastomas that showed an ORR of 63%. The median duration of response in the CNS hemangioblastoma patients was not reached and 73% exhibited a response duration of at least 12 months. In the patients with measurable pNET, the ORR was 83%, the median duration of response was not reached, and 50% of patients had a response duration of at least 12 months.4
Regarding safety of belzutifan, the most common adverse reactions reported in at least 25% of patients included laboratory findings such as decreased hemoglobin, anemia, increased creatinine, and increased glucose. Common clinical symptoms in the patients who received belzutifan were fatigue, headache, dizziness, and nausea. The most common serious adverse events occurring in Study 004 were anemia in 90% of patients (grade 3 anemia in 7%) and hypoxia in 1.6% of patients. Transfusions may be required and are recommended as clinically indicated, while erythropoiesis stimulating agents are not recommended in the setting of belzutifan-induced anemia at this time. Patient’s oxygen saturation should be monitored prior to initiation of, and periodically throughout, treatment with belzutifan. There are no dose modifications for renal or hepatic impairment, but specific dose modification recommendations are provided in the belzutifan prescribing information in patients who experience anemia, hypoxia, or other adverse reactions of grade 3 or 4 severity.4,5
Hepatic metabolism of belzutifan is primarily via UGT2B17 and CYP2C19, as well as CYP3A4 (to a lesser extent). Pharmacogenomic testing may be considered in patients with severe toxicity, as individuals who are UGT2B17, CYP2C19, or dual UGT2B17 and CYP2C19 poor metabolizers have higher drug steady state area under the curve (AUC) exposure compared with patients who are UGT2B17 normal (extensive) metabolizers and CYP2C19 nonpoor (ultrarapid, rapid, normal, and intermediate) metabolizers. As a weak CYP3A4 inducer, belzutifan may decrease serum concentrations of CYP3A4 substrates and require therapy monitoring.5
Belzutifan carries a United States (US) Boxed Warning for embryo-fetal toxicity, as exposure to the drug during pregnancy may cause embryo-fetal harm. Pregnancy status should be assessed prior to initiation. Additionally, belzutifan may render some hormonal contraceptive products ineffective and patients should be counseled on these risks and the need for effective nonhormonal contraception.5
In conclusion, understanding the genetic basis and pathogenesis of VHL disease has led to improvements in preemptive surveillance screening to help facilitate earlier diagnoses of VHL disease. Although high morbidity and mortality are associated with VHL disease, specifically in the case of multiple malignant lesions, early diagnosis and intervention may clinically impact the preservation of organ function or further disease progression. Belzutifan, a first-in-class targeted HIF-2α inhibitor, is the first FDA-approved medication for the treatment of VHL-related tumors and in the future, there is hope that other targeted agents may be found safe and effective in the treatment of patients with VHL disease.
REFERENCES
- Maher ER, Sandford RN. Von hippel-lindau disease: an update. Curr Genet Med Rep. 2019;7(4):227-235.
- Varshney N, Kebede AA, Owusu-Dapaah H, Lather J, Kaushik M, Bhullar JS. A review of von hippel-lindau syndrome. J Kidney Cancer VHL. 2017;4(3):20-29.
- Active Surveillance Guidelines. VHL Alliance. 2020. Available at: https://www.vhl.org/wp-content/uploads/2020/10/Active-Surveillance- Guidelines-2020.pdf
- Srinivasan R, Donskov F, Iliopoulos O, et al. Phase 2 study of belzutifan (MK-6482), an oral hypoxia-inducible factor 2α (HIF-2α) inhibitor, for Von Hippel-Lindau (Vhl) disease-associated clear cell renal cell carcinoma (Ccrcc). JCO. 2021;39(15_suppl):4555-4555.
- Welireg (belzutifan). Package insert. Merck & Co. Inc; 2021.
HOPA’s New Patient Advisory Panel
Chelsea Gustafson, PharmD, BCOP
Oncology Pharmacy Specialist
Community Health Network: Community Regional
Cancer Centers
Kokomo, IN
August 2021 marks the inauguration of HOPA’s new Patient Advisory Panel. This panel was developed by the Patient Outreach Committee to provide patients who have been impacted by cancer and blood diseases a platform to influence the overall mission of HOPA. The panel consists of seven cancer survivors who provide HOPA’s committees, councils, and task forces with feedback and guidance to ensure programs, initiatives, policies, and publications provide a patient perspective.
This panel is an exciting step in helping to fulfill HOPA’s vision, which is for all individuals affected by cancer to have a hematology/ oncology pharmacist as an integral member of their care team. These panelists represent a wide background of cancer types and careers; including pharmacy, information technology, research, and more.
Q&A with the Patient Advisory Panel
Each member was selected by the Patient Outreach Committee after a thorough review process. As an introduction to the panel, portions of their applications have been compiled below. For each member’s full bio, please visit the patient outreach page on hoparx.org.
Why are you interested in serving on the Patient Advisory Panel?
Morgan Kelly
The unique perspective that I have obtained by being both a patient and a chemotherapy provider (pharmacist) gives me an added ability to empathize and connect with other patients. It is a privilege to introduce myself to another patient and share that I have been in their shoes. I’m really looking forward to my involvement with the HOPA Patient Advisory Panel.
Steven Merlin
I am a nine-year survivor of metastatic pancreatic cancer. Multiple treatment regimens followed by targeted therapy in a clinical trial resulted in reaching NED status in April 2016. I am very active in patient advocacy and serve as the Outreach Chair for the New Jersey affiliate of the Pancreatic Cancer Action Network. I’ve dedicated myself to mentoring pancreatic cancer patients and caregivers with the goal of achieving the best patient outcome possible.
Thomas Henry III
I believe that my experience as a patient, coupled with my experience as a pharmacist with oncology service line experience, puts me in a unique position to understand the needs of the patient and the perspective of fellow pharmacy professionals. I look forward to working with this group.
Erin Buss
I was diagnosed with triple positive breast cancer in 2019. While a cancer diagnosis is never expected, I found myself better equipped to navigate this path because of my relationships and general understanding of the healthcare system. Because of this, I am now committed to helping to support those diagnosed after me to find their voice and advocate for themselves while making treatment decisions and beyond.
Please tell us a little about yourself.
George Jerome Valentine
I was born and raised in Philadelphia, where I lived and worked until I moved to Dallas in 1975. I started my career as an Information Technology professional in 1971 and retired in 2019. Over the years I worked in every area of Information Technology, and I have traveled and worked in numerous countries.
Mike Harrison
I am one of the first members of the Patient and Caregiver Oncology Quality Council (POQC), which was formed several years ago to provide the patient and caregiver voice for the Michigan Oncology Quality Consortium. I have also been a member of the Patient and Family Advisory Committee for the Rogel Cancer Center at Michigan Medicine for the past six years. I retired in 2016 after 29 years at Michigan Medicine, primarily as director of PR and Marketing as well as a manager in strategic planning.
Karen Fancher
I am an Associate Professor of Pharmacy Practice in Oncology Acute Care at Duquesne University School of Pharmacy in Pittsburgh. I serve on the faculty for the American Society of Health-System Pharmacists (ASHP) Board Certification in Oncology Pharmacy Review & Recertification course, as well as on various committees of both HOPA and the Advanced Topics for Oncology Pharmacy Professionals (ATOPP) Summit. My research interests include chronic leukemias and ethics in oncology.
Steven Merlin
I reside in Interlaken, a small community along the Northern New Jersey Coast. I currently split my time between the New Jersey Shore and Zürich, Switzerland. My professional career spans 35 years in academic and government research labs and the life sciences industry.
Thomas Henry III
In addition to being a CLL patient, I am a Registered Pharmacist with more than 40-years of experience. Since 2008, I have served in seventeen health systems as the Director of Pharmacy or Chief Pharmacy Officer on an interim basis.
What do you enjoy doing in your free time?
Karen Fancher
My husband Stewart and I are the proud parents of two pretty awesome teenagers. When I’m not off on an adventure with my family, you can find me reading, cooking, cheering for the Pittsburgh Steelers, and daydreaming about musical legend Sting.
Thomas Henry III
I am an avid reader, love to cook and garden. My wife, Meli, and I keep busy with entertaining and extensive travel.
Morgan Kelly
I live in North Augusta, South Carolina with my husband, our elementary-age daughter, and our two cats. Working part time enables me to enjoy time with my family, go on school field trips, and indulge in a good book or five.
How do you envision contributing as a panelist?
Steven Merlin
My focus will be to provide a patient perspective from those dealing with recalcitrant cancers, their concerns with medication side effects, costs, development, and availability time. I envision my role to serve as a liaison between HOPA and patients to translate and disseminate information, and to help patients understand the role pharmacologists and pharmacists play in patient healthcare. I’m here to help keep patients informed of advances in oncology medications and promote the importance of patients participating in clinical trials.
George Jerome Valentine
I was diagnosed with chronic lymphocytic leukemia (CLL) in 2002 and formal treatment began in 2005. I have had every test available, numerous hospitalizations, and currently take 10 different medicines daily. I feel my years of continuous treatment and dealing with doctors, hospitals and pharmacies allow me to provide unique insight to HOPA and others who are supporting or living with chronic illnesses.
HOPA is extremely fortunate to have such a wonderful group of patient panelists who have volunteered to help shape what the organization does and provides for our patients. We cannot thank this group enough for their willingness to participate and for all the insight they will bring to our organization. We look forward to seeing how this panel strengthens our mission and improves our ongoing initiatives through sharing their expertise and life perspectives. Welcome to our inaugural Patient Advisory Panel!
Safety of Inactivated Vaccines in Patients Receiving Immune Checkpoint Inhibitors
Austin Kurkowski, PharmD, BCOP
Hematology/Oncology Clinical Pharmacist
Cleveland Clinic Taussig Cancer Institute
Cleveland, OH
Grace Martin, PharmD, BCOP
Cancer Care Pharmacy Clinical Coordinator
The University of Kansas Health System
Kansas City, KS
Introduction
Immune checkpoint inhibitors upregulate the body’s immune system through T-cell activation and prevent malignant cells from evading apoptosis through 3 main mechanisms, including inhibition of programmed cell death 1 (PD-1), PD ligand 1 (PD-L1), and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4).1,2 These drugs carry a unique risk for immune-related adverse events (irAEs) which can manifest in many organ systems.
The most common irAEs include rash, pruritus, colitis, hepatitis, pneumonitis, hypo- or hyperthyroidism, hypophysitis, and adrenal insufficiency. These adverse events vary in severity and may lead to treatment delay or discontinuation. One meta-analysis described an incidence of grade ≥3 irAEs between approximately 7% and 55%.3 It is difficult to predict which patients will have irAEs, and when they may occur. New antigen exposure from inactivated vaccines allows for T-cell interactions that may drive increased irAEs.4,5
Literature describing the incidence and severity of irAEs with the use of inactivated vaccines is limited and only describes the risks associated with influenza vaccination. In 2018, one study showed that patients receiving PD-1 inhibitor therapy concurrent with inactivated influenza vaccines had a higher incidence of grade 3 or 4 irAEs than historical controls.6 More recent studies, however, contradicted these data.4,7 Few primary studies evaluated inactivated vaccines other than influenza vaccines concurrently with immunotherapy. There are limited published data of vaccines in patients who are receiving immunotherapy beyond nivolumab or pembrolizumab, or in combination with chemotherapy.4,6-8
Methods
At the University of Kansas Health System, various inactivated vaccines are administered to patients who are receiving immune checkpoint inhibitor therapy. Currently, no institutional policies are in place for the use of inactivated vaccines in patients who are receiving immunotherapy. This study attempted to determine the relative incidence rate of irAEs requiring therapeutic intervention in the vaccinated versus a control group. We also assessed the rates of therapy delay or discontinuation due to irAEs. A therapeutic intervention for an irAE was defined as a delay of immune checkpoint inhibitor treatment by ≥14 days after the expected date of therapy for cycle 2 or beyond, discontinuation of treatment, or the addition of supportive therapy. Supportive therapy was defined as the addition of a medication for the management of irAEs, such as corticosteroids or other immunosuppressants. Only irAEs that newly occurred after receipt of a vaccine and an immune checkpoint inhibitor were included.
Study Population and Results
This study included a total of 213 patients; 71 in the vaccinated cohort and 142 in the control cohort (1:2 vaccinated to control matching was done based on age and immune checkpoint inhibitor received). Patients in the vaccinated cohort were required to have received an inactivated vaccine 30 days before or 60 days after the administration of immune checkpoint inhibitor. Patients were reviewed for demographics (age, race, gender), malignancy, and type of vaccine and immunotherapy.
The most common malignancies included non-small-cell lung cancer, melanoma, renal-cell carcinoma, small-cell lung cancer, and head and neck cancer. The inactivated influenza vaccine was the most frequently (N = 48) administered, followed by pneumococcal vaccines (PPSV23, N = 19; PCV13, N = 8) and the Tdap vaccine (N = 8). Other vaccines included inactivated polio, recombinant zoster, and hepatitis A. Twelve patients received more than 1 type of inactivated vaccine. Those in the vaccinated cohort most often received nivolumab monotherapy (40.8%) or pembrolizumab monotherapy (35.2%); fewer patients received nivolumab plus ipilimumab (5.6%), nivolumab plus chemotherapy (2.8%), or pembrolizumab plus chemotherapy (9.9%), or other therapies (5.7%).
A total of 22.5% (N = 16) of vaccinated patients had an irAE requiring an intervention versus 26.8% (N = 38) of patients in the control cohort (P = .50). No significant differences were observed in the rates of therapy delays resulting from irAEs (vaccinated 9.9% vs control 9.2%; P = .87) or discontinuations resulting from toxicity (vaccinated 9.9% vs control 9.9%; P = 1.00). All patients requiring the use of supportive therapy received a corticosteroid, and no difference was seen in the rate of patients requiring additional therapy between the 2 cohorts (vaccinated, 18.3%; control, 25.4%; P = .25). Common irAEs requiring intervention included rash or pruritus, pneumonitis or dyspnea, diarrhea or colitis, and transaminitis.
Patients who started immune checkpoint inhibitor therapy before vaccination had a median time from vaccination to intervention of 60 days (range, 0-368 days). In patients who were vaccinated first, the median time from initiation of immunotherapy to intervention was 74.5 days (range, 14-86 days). For the 1 patient who received a vaccine on the same day that immunotherapy was initiated, the time to intervention was 71 days.
Discussion and Key Takeaways
Expanding the scope of understanding how various inactivated vaccines and immunotherapy work in concert to cause irAEs will give clinicians an increased knowledge needed to engage in informed medical decision-making. This study indicates that patients may safely receive immune checkpoint inhibitors with inactivated vaccines, and increased receipt of appropriate vaccines can provide optimized care to an already vulnerable patient population.
Our study used a more stringent time frame than previous studies, limiting the receipt of a vaccine to only 30 days before an immune checkpoint inhibitor administration.4 We allowed for vaccination up to 60 days after immune checkpoint inhibitor administration, given the long half-life of these medications. It was also notable that no patients required nonsteroidal immunosuppressive therapy, such as infliximab. We therefore presumed that none of the patients’ irAEs were refractory to their management.
A noteworthy but unexpected irAE in two patients who received vaccines was graft-versus-host disease (GVHD) reactivation after the initiation of immune checkpoint inhibitor therapy, which followed allogeneic stem-cell transplant. A total of four patients in the vaccinated cohort had a history of allogeneic stem-cell transplant. These patients were not excluded, to facilitate a wholistic and real-world perspective. The high incidence of GVHD in this setting has been described in previous studies.9,10 In both cases, the complex clinical scenario made it difficult to definitively correlate acute GVHD with immune checkpoint inhibitor therapy, but the medications may be contributing factors, given the acute changes in clinical condition and proximity to receipt of immune checkpoint inhibitor therapy.
To our knowledge, this study is one of the first studies to include data of inactivated vaccines beyond influenza and with regimens combining traditional chemotherapy and checkpoint inhibitor therapy. The results in our study are congruent with a 2017 study that showed that routine vaccination in patients receiving immunotherapy did not increase the number or severity of adverse events.11 This is the only other study that included data on a vaccine other than influenza.
Future Directions and Conclusion
The risk for patients who are not vaccinated may significantly outweigh the risk for irAEs. Future studies could include measuring the incidence of vaccine-preventable infections and its impact on patients’ morbidity and mortality. The pharmacoeconomic impact of patients who are not receiving appropriate vaccinations should also be considered.
In this study, immune checkpoint inhibitor treatment delays and discontinuations were minimal and similar between patients who were vaccinated and those who were not, indicating that patients who are receiving such treatment should not expect to have their duration of therapy influenced by the administration of an inactivated vaccine. Providers should therefore continue to recommend inactivated vaccines to the appropriate patients.
To our knowledge, this is one of few studies that provides information on the use of inactivated vaccines beyond influenza. Our findings confirm that vaccination is a safe, guideline-recommended practice.
REFERENCES
- Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86.
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561.
- El Osta B, Hu F, Sadek R, et al. Not all immune-checkpoint inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol. 2017;119:1-12.
- Chong CR, Park VJ, Cohen B, et al. Safety of inactivated influenza vaccine in cancer patients receiving immune checkpoint inhibitors. Clin Infect Dis. 2020;70:193-199.
- Finnefrock AC, Tang A, Li F, et al. PD-1 blockade in rhesus macaques: impact on chronic infection and prophylactic vaccination. J Immunol. 2009;182:980-987.
- Läubli H, Balmelli C, Kaufmann L, et al. Influenza vaccination of cancer patients during PD-1 blockade induces serological protection but may raise the risk for immune-related adverse events. J Immunother Cancer. 2018;6:40.
- Wijn DH, Groeneveld GH, Vollaard AM, et al. Influenza vaccination in patients with lung cancer receiving anti–programmed death receptor 1 immunotherapy does not induce immune-related adverse events. Eur J Cancer. 2018;104:182-187.
- Gwynn ME, DeRemer DL, Saunders KM, et al. Immune-mediated adverse events following influenza vaccine in cancer patients receiving immune checkpoint inhibitors. J Oncol Pharm Pract. 2020;26:647-654.
- Herbaux C, Gauthier J, Brice P, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood. 2017;129:2471-2478.
- Haverkos BM, Abbott D, Hamadani M, et al. PD-1 blockade for relapsed lymphoma post–allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood. 2017;130:221-228.
- Schenk EL. Clinical outcomes of patients on check point inhibitor therapy who receive routine vaccinations. J Clin Oncol. 2017;35(15_suppl):Abstract e14597.
Genentech’s Withdrawal of the Indication for Atezolizumab in Triple Negative Breast Cancer
Christine Barrett, PharmD
Clinical Pharmacy Specialist in Hematology/Oncology
Allegheny General Hospital
Pittsburgh, PA
Breast cancer is the most common cancer diagnosed in women in the United States.1 Triple-negative breast cancer (TNBC) is an aggressive subtype that accounts for nearly one-fifth of all cases. It is defined by a lack of expression of both estrogen (ER) and progesterone (PR) receptors, as well as human epidermal growth factor receptor-2 (HER2). TNBC is associated with an earlier age of onset, higher rates of recurrence, and poorer prognosis in comparison to hormone-sensitive subtypes.2 Because it is not sensitive to endocrine therapy or HER2-targeted therapies, there are limited therapeutic options, and chemotherapy has been the standard for systemic treatment of TNBC.2-3
As seen in many other types of cancer, immunotherapy has become an emerging treatment option in breast cancer. In comparison to other subtypes, TNBC is associated with higher levels of programmed cell death ligand 1 (PD-L1) expression. PD-1 and its ligand PD-L1 are immune checkpoint receptors expressed on activated T-cells and other immune cells; higher levels of expression of these receptors are associated with tumor immune resistance.1 In addition, TNBC is also associated with increased genomic instability, and higher enrichment by tumor-infiltrating lymphocytes (TILs). These are all indications that TNBCs may be more immunogenic and therefore more responsive to immunotherapy.3
Atezolizumab is a humanized IgG1 antibody targeting PD-L1, which prevents its interaction with receptors PD-1 and B7-1, resulting in a reversal of T-cell suppression and therefore restoring antitumor T-cell activity.4 In March 2019, atezolizumab received accelerated approval from the US Food and Drug Administration (FDA) for use in combination with nab-paclitaxel in patients with unresectable locally advanced or metastatic TNBC whose tumors express PD-L1. Atezolizumab was the first immune checkpoint inhibitor to receive approval in metastatic TNBC.3 The accelerated approval was based on preliminary results of progression-free survival in the IMpassion130 trial, and was contingent on the confirmatory results of the subsequent IMpassion131 study.3
The IMpassion130 trial enrolled 902 patients with previously untreated metastatic TNBC. Patients were randomized to receive atezolizumab 840 mg or placebo on days 1 and 15 in combination with nab-paclitaxel 100 mg/m2 on days 1, 8, and 15 every 28 days for six cycles or more. Co-primary endpoints were investigator-assessed progression-free survival and overall survival in both the intent-to-treat and PD-L1-positive subgroup, which comprised 40.9% of patients. PD-L1 positivity was defined as ≥ 1% expression on tumor-infiltrating immune cells. A statistically significant improvement in median progression-free survival was seen in both the intent-to-treat group (7.2 months versus 5.5 months; hazard ratio [HR] for progression or death 0.8, 95% confidence interval [CI] 0.69 to 0.92, p=0.002) and in the PD-L1-positive subgroup (7.5 months versus 5 months; HR 0.62, CI 0.49 to 0.78, p<0.001). There was a numerical improvement in median overall survival in the intent-to-treat group (21.3 months versus 17.6 months; HR 0.84, CI 0.69 to 1.02, p=0.08), however it was not statistically significant. Overall survival was also numerically improved in the PD-L1-positive subgroup (25 months versus 15.5 months); due to the hierarchical testing procedure of the study, statistical significance was not determined.
Results of the final overall survival analysis of IMpassion130 were recently published; the difference in median overall survival was still unable to reach statistical significance (21 months versus 18.7 months; HR 0.87, CI 0.75 to 1.02, p=0.077). In an exploratory analysis in the PD-L1-positive subgroup, the median overall survival was 25.4 months in the atezolizumab group in comparison to 17.9 months in the control group.6
The subsequent IMpassion131 was conducted in 651 patients with previously untreated metastatic TNBC. In this study, patients were randomized to receive atezolizumab 840 mg or placebo on days 1 and 15 in combination with paclitaxel 90 mg/m2 on days 1, 8, and 15 every 28 days for six cycles or more. The primary endpoint was investigator-assessed progression-free survival, which also followed hierarchical testing, but was tested first in the PD-L1-positive subgroup, which made up 45% of the study population. The IMpassion131 found that progression-free survival was not significantly improved with atezolizumab in either the PD-L1-positive subgroup (6 months versus 5.7 months; HR 0.82, CI 0.60 to 1.12, p=0.20) or the intent-to-treat population (5.7 months versus 5.6 months). The combination also did not improve overall survival in the PD-L1-positive subgroup (22.1 months versus 28.3 months) or the intent-to-treat population (19.2 months versus 22.8 months).7
Due to the unfavorable results of the IMpassion131, continued approval of atezolizumab in metastatic TNBC was discussed by the FDA Oncology Drugs Advisory Committee (ODAC) in April 2021. Although the committee voted to maintain the accelerated approval, after further evaluation by the FDA and the manufacturer Genentech, the manufacturer voluntarily withdrew the accelerated approval for atezolizumab in combination with nab-paclitaxel in metastatic TNBC on August 27, 2021.8
There have been several proposed reasons for the discrepancies between the results of IMpassion130 and IMpassion131, such as differences in baseline characteristics, the use of different taxanes, and the role of steroids. Differences in study populations with respect to tumor cell biology and other molecular markers were not collected in either study, which could affect patients’ overall prognosis and response to therapy. Other theories include a possible difference in immunogenic effects between nab-paclitaxel and paclitaxel and also a dampening of immunotherapy effects in combination with steroid pre-medications, although there is now data from the KEYNOTE-522 and KEYNOTE-355 studies which support the use of both nab-paclitaxel and paclitaxel in combination with immunotherapy.9-12
The results of the IMpassion131 and the withdrawal of atezolizumab’s indication in combination with nab-paclitaxel highlight the ongoing challenges with immunotherapy in metastatic TNBC. Currently, there is an approval for immunotherapy in metastatic TNBC; this approval is with pembrolizumab in combination with chemotherapy in patients whose tumors express PD-L1 with a combined positive score (CPS) ≥ 10, based on the results of the KEYNOTE-355 study.12 Future research in immunotherapy for metastatic TNBC should focus on developing more reliable predictive biomarkers and identifying patient populations more likely to benefit from immunotherapy.
REFERENCES
- Surveillance, Epidemiology, and End Results Program (SEER). Cancer Stat Facts: Cancer of Any Site. https://seer.cancer.gov/statfacts/html/all.html. Published 2021. Accessed October 10, 2021.
- Berger ER, Park T, Saridakis A, Golshan M, Greenup RA, Ahuja N. Immunotherapy treatment for triple negative breast cancer. Pharmaceuticals. 2021;14(8):763.
- Kwapisz D. Pembrolizumab and atezolizumab in triple-negative breast cancer. Cancer Immunol Immunother. 2021;70(3):607-617.
- Tecentriq (atezolizumab) [prescribing information]. South San Francisco, CA: Genentech Inc; April 2021.
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. New England Journal of Medicine. 2018;379(22):2108-2121.
- Emens LA, Adams S, Barrios CH, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol. 2021;32(8):983-993.
- Miles D, Gligorov J, André F, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/ metastatic triple-negative breast cancer. Ann Oncol. 2021;32(8):994-1004.
- Genentech. Genentech Provides Update on Tecentriq U.S. Indication for PD-L1-Positive, Metastatic Triple-Negative Breast Cancer. https://www.gene.com/media/press-releases/14927/2021-08-27/genentech-provides-update-on-tecentriq-u. Published 2021. Accessed September 24, 2021.
- Franzoi MA, Azambuja E de. Atezolizumab in metastatic triple-negative breast cancer: IMpassion130 and 131 trials - how to explain different results? ESMO Open. 2020;5(6).
- Voorwerk L, Kok M. ‘IMpassionate conflicts’ in immunotherapy trials for metastatic triple-negative breast cancer. Annals of Oncology. 2021;32(8):947-949.
- Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. New England Journal of Medicine. 2020;382(9):810-821.
- Cortes J, Cescon DW, Rugo HS, et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. JCO. 2020;38(15_ suppl):1000-1000.
