Feature: Summary of Hepatitis B Virus Screening and Management for Patients with Cancer
Lindsay Orton, PharmD
PGY-2 Oncology Pharmacy Resident Vanderbilt University Medical Center
Nashville, TN
It is well known that patients with hematologic malignancies who receive anti-CD20 monoclonal antibodies or undergo a stem cell transplantation are at a high risk of hepatitis B virus (HBV) infection. However, the risk of other anticancer therapies and cancer types (i.e. solid tumors) are not as well understood.
American Society of Clinical Oncology (ASCO)’s 2015 provisional clinical opinion (PCO) recommended screening for HBV infection only in patients who start anti-CD20 therapy, undergo a stem cell transplantation, or have risk factors for HBV. These risk factors include persons born in countries/regions with a HBV infection prevalence >2%, United States-born persons not vaccinated as infants whose parents were born in regions with HBV infection prevalence >8%, HIV-positive persons, IV drug users, men who have sex with men, and persons with household or sexual contact with persons with HBV infection.1 ASCO has published an updated 2020 PCO.2 (Please see below.)
In accordance with the American Association for the Study of Liver Diseases (AASLD), the expert panel defined HBV reactivation and hepatitis flare as outlined below.3
- HBV reactivation from chronic HBV: 100-fold increase in HBV DNA compared with baseline, HBV DNA >1000 IU/mL if previously undetectable, or HBV DNA >10,000 IU/mL if baseline HBV DNA not available
- HBV reactivation from past HBV: detectable HBV DNA and reverse seroconversion of hepatitis B surface antigen (negative to positive)*
- Hepatitis flare: Alanine transaminase (ALT) increase >3x baseline and >100 U/L
*To simplify guidance, the 2020 PCO uses a cut-off threshold of HBV DNA >1000 IU/mL to warrant further management in patients with past HBV infection.
Recommendations
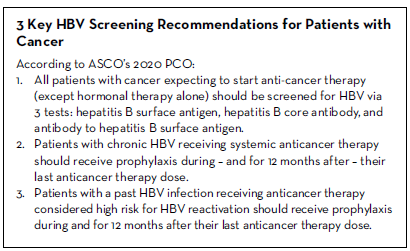
1. All patients with cancer expecting to start anti-cancer therapy (except hormonal therapy alone) should be screened for HBV via three tests: hepatitis B surface antigen, hepatitis B core antibody, and antibody to hepatitis B surface antigen.
This recommendation represents the most significant change from the 2015 PCO. There have been several large, prospective clinical trials that have supported universal HBV screening compared to HBV risk-based screening. Brasseur et al. analyzed a survey of potential HBV infection risk factors in 388 patients with a solid tumor. The investigators found moderate sensitivity and specificity (46% and 56%) of the risk factor questions, however, a very poor positive predictive value (9%).4 Based on these results, use of an HBV risk factor approach for screening was discouraged.
Hwang et al. analyzed a 5-7 item and a 19-item HBV risk survey in 2,124 patients with a hematologic malignancy or solid tumor. Regardless of the number of items, the surveys resulted in high sensitivity (99-100%), but low specificity (<15%), which was attributable to the high likelihood of patients having at least 1 risk factor (i.e. older age, black or Asian race).5 Based on these survey results, almost 90% of patients would meet the criteria to undergo HBV serology testing, indicating that selective HBV screening is impractical and therefore universal screening is recommended.
A positive hepatitis B surface antigen (HBsAg) indicates chronic HBV infection whereas a positive hepatitis B core antibody (anti-HBc) demonstrates past HBV infection. However, chronic HBV patients will often have a positive anti-HBc, as well. Either a total immunoglobulin (IgM and IgG) or IgG should be used for anti-HBc testing. Positive IgM confers an acute infection and is therefore not recommended to be used alone for screening. The third test, antibody to hepatitis B surface antigen (anti-HBs), indicates immunity. For patients with past HBV infection (anti-HBc positive), an associated positive anti-HBs represents a resolved infection whereas a negative anti-HBs indicates an isolated core. A positive anti-HBs with negative HBsAg and anti-HBc correlates with vaccine-induced protective immunity.
Anti-HBc positivity likely reduces the risk of HBV reactivation in patients with past HBV. A meta-analysis of 1,672 patients with past HBV (anti-HBc positive) identified a 14% reactivation risk when anti-HBs was negative compared to a 5% risk when it was positive.6 To note, HBV tests should be interpreted with caution in patients that have received IVIG, which has been shown to passively transfer anti-HBc leading to false-positive results. Therefore, patients should ideally be screened for HBV prior to receiving IVIG.
The panel recommends screening all patients prior to, or at the start of, anticancer therapy, however, the results of the screening test should not delay therapy. An exception to this recommendation is patients who receive hormonal therapy alone without systemic anticancer therapy. Hormonal therapy alone is unlikely to increase the risk of HBV reactivation in patients with chronic or past HBV and therefore, these patients do not require upfront HBV screening. However, patients that receive steroids in addition to their hormonal therapy (i.e. abiraterone plus prednisone) could be at increased risk for HBV reactivation and therefore screening should be considered. In addition, if their regimen changes to include any additional anticancer therapy beyond hormonal therapy, patients should be screened for HBV prior to initiation or at the start of this new regimen.
2. Patients with chronic HBV receiving systemic anticancer therapy should receive prophylaxis during – and for 12 months after – completion of their anticancer therapy.
Chronic HBV can lead to cirrhosis, liver failure, and hepatocellular carcinoma (HCC). The AASLD 2018 hepatitis B guidance reported approximately a 50% risk of HBV reactivation for patients with chronic HBV and a hematologic malignancy.3 Similarly, patients with HCC and chronic HBV are at increased risk for reactivation. Patients with other solid tumors and chronic HBV are also at a heightened risk, however, the data for HBV reactivation associated with the anticancer regimens used for these patients are limited. Until more data are available, the panel recommends that all of these patient groups receive antiviral prophylaxis starting before, continued during, and for 12 months after, their anticancer therapy if they are HBsAg positive.
There are currently 3 preferred medications for HBV prophylaxis because of their high potency and high viral resistance barrier: entecavir 0.5 mg daily, tenofovir disoproxil fumarate (TDF) 300 mg daily, or tenofovir alafenamide (TAF) 25 mg daily. However, due to their ability to suppress replication but not eliminate the viral genome, long-term therapy is required. Given their additional anti-HIV properties, prior to starting entecavir, TDF or TAF, patients should be tested for HIV. Monotherapy is not recommended for patients who test HIV positive.
While on antiviral prophylaxis, patients should be monitored by checking alanine aminotransferase (ALT) and HBV DNA level at baseline and every 6 months during their antiviral therapy. Due to the risk of hepatitis flares, ALT should be monitored monthly for the first 3 months after discontinuation of antiviral therapy, and every 3 months thereafter.
3. Patients with a past HBV infection receiving anticancer therapy considered high risk for HBV reactivation should receive prophylaxis during – and for 12 months after – their last anticancer therapy dose.
Patients with past HBV still contain HBV DNA in their liver and are susceptible to reactivation only during potent immunosuppression. In contrast to chronic HBV, patients with past HBV (both resolved infection and isolated core) only require antiviral prophylaxis if they are considered high risk for reactivation, which includes receiving an anti-CD20 monoclonal antibody or stem cell transplantation. Similar to chronic HBV, these high-risk patients will receive antiviral prophylaxis with entecavir, TDF or TAF during and for 12 months after completion of their anticancer therapy.
Longer antiviral therapy may be warranted given the increased risk of reactivation for nearly 2 years following completion of anticancer therapy, especially in those that are anti-HBs negative, which confers a higher reactivation risk compared to anti-HBs positive patients. Seto et al. found a significantly higher rate of reactivation associated with anti-HBs negativity compared to positivity (68.3% vs 34.4%, p=0.012) among 260 patients receiving rituximab-containing chemotherapy.7 During antiviral therapy, monitoring includes HBV DNA and ALT obtained at baseline and every 6 months.
Alternatively, patients may undergo close monitoring with HBsAg and HBV DNA every 3 months instead of pre-emptively initiating antiviral prophylaxis in all high-risk anti-HBc positive patients. In this approach, antiviral prophylaxis should be promptly started at the earliest sign of HBV reactivation (reverse HBsAg seroconversion or HBV DNA >1000 IU/mL). Seto et al. analyzed 83 past HBV patients receiving anti-CD20 therapy. Patients were monitored monthly without antiviral prophylaxis, which resulted in a 25% reactivation rate. Upon reactivation, patients were started on antiviral therapy and monitoring increased to every 2 weeks. All patients that received antiviral therapy had normalization of ALT and a return of HBV DNA to undetectable levels.8 If HBV DNA is quantifiable but still <1000 IU/mL, monthly monitoring of HBV DNA may be warranted. Upon initiation of antiviral therapy, ALT should be checked at baseline and every 6 months while on therapy. Following discontinuation, ALT should be monitored monthly for the first 3 months and then every 3 months thereafter. This alternative approach may be considered for patients that are adherent to close and frequent follow-up, including the 12 months following completion of anticancer therapy given the risk of delayed reactivation.
Other Considerations
Vaccination
Patients who are negative for all 3 screening tests (HBsAg, anti-HBc and anti-HBs) and have never been exposed to HBV, are not immune, and therefore are susceptible to HBV infection. Vaccination may be recommended, however, there is insufficient data on vaccinating immunocompromised patients and modified dosing regimens (i.e. doubling the dose or administering additional doses) may be warranted given the reduced humoral response in these patients. Some research suggests waiting 3-6 months following cessation of anticancer therapy to administer vaccines and 1-2 months following the final dose of the HBV vaccine series to test for anti-HBs.9,10
Cost
Financial burden for patients is a huge barrier to implementing these recommendations. Various cost effectiveness analyses have found a benefit for universal screening and antiviral prophylaxis for patients with hematologic malignancies at high risk for HBV reactivation, however, the analyses for solid tumor patients at lower risk for reactivation have been conflicting. More research on the risk of HBV reactivation for solid tumor patients is needed to determine the cost effectiveness of universal screening and prophylaxis.
In summary, the expert panel recommends screening all cancer patients for HBV prior to starting anticancer therapy, and initiating antiviral prophylaxis in all patients found to have chronic HBV or high-risk patients found to have past HBV.
REFERENCES
- Hwang J, Somerfield M, Feld J, et al. Hepatitis B virus screening for patients with cancer before therapy: American Society of Clinical Oncology provisional clinical opinion update. J Clin Oncol. 2015;32:2212- 2220.
- Hwang J, Feld J, Hammond S, et al. Hepatitis B virus screening and management for patients with cancer prior to therapy: ASCO provisional clinical opinion update. J Clin Oncol. 2020;38:3698-3715.
- Terrault N, Lok A, McMahon B, et al. Update on prevention, diagnosis and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599.
- Brasseur M, Heurgué-Berlot A, Barbe C, et al. Prevalence of hepatitis B and C and sensibility of selective screening questionnaire in patients receiving chemotherapy for solid tumors. BMC Cancer. 2015;15:999.
- Hwang J, Lok A, Fisch M, et al. Models to predict hepatitis B virus infection among patients with cancer undergoing systemic anticancer therapy: A prospective cohort study. J Clin Oncol. 2018;36:959-967.
- Paul S, Dickstein A, Saxena A, et al. Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: A meta-analysis. Hepatology. 2017;66:379-388.
- Seto W, Chan T, Hwang Y, et al. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol. 2014;32:3236-3743.
- Seto W, Chan T, Hwang Y, et al. Monitoring and treatment of patients undergoing immunotherapy with anti-CD20 who are exposed to HBV. Clin Gastroenterol Hepatol. 2019;17:1410-1412.
- Schille S, Vellozzi C, Renigold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2018;67:1-31.
- Rubin L, Levin M, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44-e100.
