Brief Update on Newly Approved Agents for the Treatment of Chronic Lymphocytic Leukemia
Ashley Glode, PharmD BCOP
Clinical Oncology Pharmacy Specialist
PGY2 Oncology Residency Program Director
Adjunct Assistant Professor—Clinical Pharmacy and Outcomes Sciences Medical University of South Carolina/South Carolina College of Pharmacy Charleston, S
Megan V. Brafford, PharmD BCOP
Clinical Oncology Pharmacy Specialist Baptist Health Lexington
Lexington, KY
Chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) accounts for approximately 7% of newly diagnosed cases of non-Hodgkin’s lymphoma (NHL)1 and is the most common leukemia diagnosed in the Western world.2 CLL and SLL basically are the same disease and are treated similarly (unless otherwise indicated, however, this article will focus on SLL). With CLL, the disease burden primarily is in the bloodstream and bone marrow, and with SLL, the lymph nodes are involved.3 In the United States, 15,720 new diagnoses and 4,600 new deaths from CLL are predicted to occur in 2014.4 CLL is considered an indolent NHL with median age at diagnosis of 72 years.5 Signs and symptoms of this malignancy are vague and include weakness, weight loss, fever, night sweats, enlarged lymph nodes, and early satiety, but patients also may be asymptomatic when they are diagnosed.6
Diagnosis typically involves evaluation of the patient’s complete blood count (CBC) with differential, peripheral blood smear, immunophenotype of the circulating lymphocytes, and a thorough physical exam. Molecular cytogenetics are also performed to assess for specific gene mutations, such as deletion 11q or 17p, which are poor prognostic indicators. Unmutated IgVH (immunoglobulin heavy chain variable region) and high expression of Zap70 or CD38 also are poor prognostic factors. Bone-marrow biopsies are not required for diagnosis of CLL but may be completed in select patients. Excisional lymph node biopsies are required for the diagnosis of SLL.3,6,7
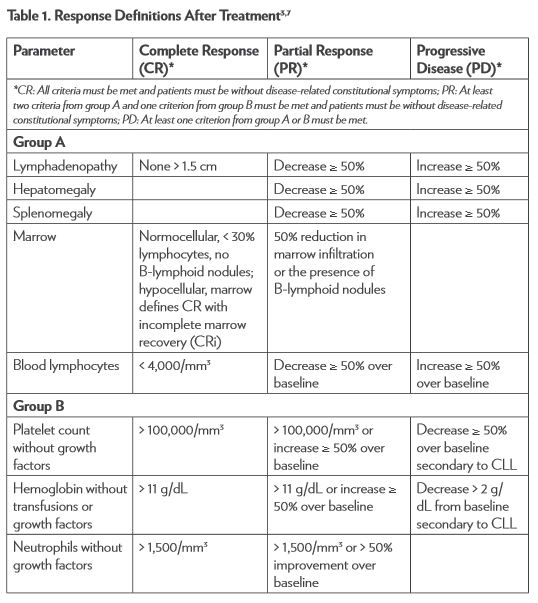
The National Cancer Institute–sponsored Working Group (NCI-WG) on CLL published revised guidelines for the diagnosis and management of this malignancy in 2008.7 To determine response to therapy (Table 1), assessment must include physical examination and evaluation of blood parameters. Response assessments should be conducted at least 2 months after treatment is completed. Stable disease (SD) is when patients do not have progressive disease (PD) but do not meet the criteria for complete response (CR) or partial response (PR). Relapse is described as evidence of disease progression after 6 or more months following an initial CR or PR. Refractory disease is expressed as failure to achieve a response or having disease progression within 6 months of the last treatment.3,7
Treatment Options
Treatment options for CLL have progressed during the past several decades, particularly in recent years. Several ongoing clinical trials are evaluating the efficacy of novel drug combination regimens and agents targeting unique pathways in B-cell malignancies. Treatment of this NHL subtype ranges from close observation with supportive-care measures to a variety of more intense therapeutic options. CLL is generally incurable, occurs in older patients, and progresses slowly. Therefore, it is often treated conservatively with careful consideration of the patient’s performance status and comorbidities.3,8
Patients who are asymptomatic may be observed but not treated until they become symptomatic, whereas patients with significant disease-related symptoms should be treated. Several pieces of clinical information should be considered if a patient is to be treated for CLL. Age, comorbidities, performance status, and presence of specific chromosomal abnormalities and gene mutations all should be evaluated when electing treatment regimens on an individual basis. Enrollment in a clinical trial should always be considered.3,6 Despite numerous available treatment options, some patients may be refractory to therapy, needing alternative treatment options, or require allogeneic hematopoietic stem cell transplantation (HSCT) for disease control. New agents have recently been added to the treatment armamentarium, which has improved patient options and prolonged survival.
Obinutuzumab (Gazyva)
Obinutuzumab is a humanized, glycoengineered IgG1 type 2 antibody targeted against CD20. Obinutuzumab has high-affinity binding for the type 2 epitope leading to 5- to 100-fold greater antibody-dependent cytotoxicity than rituximab.8,9 Obinutuzumab was approved by the U.S. Food and Drug Administration (FDA) in November 2013 for use in combination with chlorambucil for the treatment of patients with untreated CLL.10
Obinutuzumab was studied in a multinational trial (189 centers in 26 countries) that enrolled untreated patients with CD-20 positive CLL, Binet stage C, or symptomatic disease. Patients were also required to have a clinically meaningful burden of coexisting conditions, defined as a score higher than 6 on the Cumulative Illness Rating Scale (CIRS). Patients who did not have a high enough comorbidity score were also eligible if they had a creatinine clearance of 30–69 mL/min calculated with the Cockcroft-Gault formula. This open-label, three- group study randomized patients in a 1:2:2 manner: (a) chlorambucil alone, (b) obinutuzumab plus chlorambucil, and (c) rituximab plus chlorambucil, respectively; all regimens were given in six 28-day cycles. Chlorambucil was administered at 0.5 mg/ kg orally once on days 1 and 15. Obinutuzumab was administered at 1,000 mg intravenously on days 1, 8, and 15 during cycle 1, then only on day 1 during all subsequent cycles. The first infusion was divided over 2 days for cycle 1 after a protocol amendment to help decrease the rates of infusion reactions. Rituximab was administered at 375 mg/m2 intravenously on day 1 during cycle 1, then 500 mg/m2 during all subsequent cycles.9
After 118 patients had been assigned to the chlorambucil arm, this arm was closed early due to predefined stopping criteria. The protocol was revised to randomize patients in a 1:1 ratio into the remaining arms stratified by geographic region and stage. Patients in the single-agent chlorambucil arm were allowed to cross over to the obinutuzumab- plus-chlorambucil arm if they had PD during treatment or within 6 months of the end of treatment.9
A total of 781 patients were enrolled in the three study arms. Baseline characteristics among the three groups were well balanced, with a median age of 73 years, creatinine clearance of 62 mL/min, and a CIRS score of 8. The site investigator determined that progression- free survival (PFS) was the primary end point. There was significant improvement in PFS for the combination arms over the arm receiving chlorambucil alone—26.7 months for obinutuzumab plus chlorambucil versus 11.1 months for chlorambucil alone (hazard ratio [HR] = 0.18; 95% confidence interval [CI]: 0.13-0.24; p < .001) and 16.3 months for rituximab plus chlorambucil (HR = 0.44; 95% CI: 0.34-0.57; p < .001). Patients with deletion 17p were the only subgroup who did not experience this benefit in PFS. PFS was also significantly longer when obinutuzumab plus chlorambucil was compared with rituximab plus chlorambucil: 26.7 versus 15.2 months (HR = 0.39; 95% CI: 0.31-0.49; p <.001) . Additionally, obinutuzumab in combination with chlorambucil resulted in higher rates of overall, complete, and molecular responses.
At the time of publication, the most recent data for overall survival (OS) revealed a significant improvement for the obinutuzumab plus chlorambucil arm over the chlorambucil monotherapy arm; 9% versus 20% (HR for death= 0.41; 95% CI: 0.23-0.74; p = 0.002). There was no significant OS difference in the combination therapy arms.9
Adverse reactions occurred most commonly in the obinutuzumab plus chlorambucil arm, including neutropenia, anemia, thrombocytopenia, leukopenia, and infusion-related reactions. Infection grade 3–5 ranged from 11% to 14% and was not significantly different between groups, with the majority of infections being bacterial in nature. Twenty percent of patients experienced grade 3–4 infusion reactions with the first infusion of obinutuzumab, yet no grade 3–4 reactions occurred during subsequent cycles. Patients in the rituximab plus chlorambucil arm were the least likely of all groups to discontinue therapy early due to adverse events. The primary reason for discontinuation in the obinutuzumab plus chlorambucil group was infusion-related reactions, which decreased with the divided dosing on day 1 of cycle 1 (100 mg on day 1 and 900 mg on day 2).9
Obinutuzumab plus chlorambucil has been added to the National Comprehensive Cancer Network (NCCN) guidelines as a preferred treatment option for first-line therapy of CLL.3 The infusion-related adverse reactions are manageable with appropriate premedications of acetaminophen, antihistamine, and corticosteroid.9 Obinutuzumab is being evaluated in the relapsed/refractory setting as well as in various combinations in both the relapsed/refractory and untreated setting.11
Ibrutinib (Imbruvica)
Ibrutinib is an oral agent that inhibits Bruton’s tyrosine kinase (BTK). This enzyme target is essential for B-cell receptor signaling, proliferation, and survival.12,13 The FDA approved ibrutinib in February 2014 for the treatment of patients with CLL who have received at least one previous therapy.13 In July 2014, it was also approved for treatment of patients with deletion 17p CLL.14
Ibrutinib was evaluated in RESONATE, a phase 3, multicenter, open- label, randomized trial that enrolled patients with relapsed or refractory CLL or SLL. RESONATE compared ibrutinib, 420 mg orally once per day, with ofatumumab, 300 mg intravenously week 1 followed by 2,000 mg intravenously weekly for 7 weeks, then every 4 weeks for 16 weeks. From 67 sites in the United States, Australia, and seven European countries, 391 patients were stratified according to purine analog chemoimmunotherapy resistance and presence of 17p13.1 deletion. Due to positive results from the phase 2 trial with ibrutinib, the trial was revised to allow crossover of patients from ofatumumab to ibrutinib.13
The baseline characteristics of patients were well matched between the two groups. Patients in the ibrutinib group received a median of 8.6 (0.2–16.1) months of therapy, and patients in the ofatumumab group received 5.3 (0–7.4) months of therapy. The primary end point of PFS was significantly prolonged in the ibrutinib group: 9.4 months versus 8.1 months for the ofatumumab group (HR for progression or death = 0.22; 95% CI: 0.15–0.32; p < .001 by log-rank test). Ibrutinib’s impact on PFS was seen regardless of baseline clinical characteristics or molecular features. OS was also significantly prolonged in the ibrutinib arm (HR = 0.43; 95% CI: 0.24-0.79; p = .005). The improvement in OS was maintained in all subgroups according to the pretreatment and genetic abnormalities.13
Lymphocytosis occurred in 69% of patients in the ibrutinib arm and was not considered disease progression. This lymphocytosis is a result of the lymphocytes leaving the nodal compartments and resolves within 8 months in most patients.15 The most common nonhematologic adverse events occurring in at least 20% of patients were diarrhea, fatigue, pyrexia, and nausea in the ibrutinib arm and fatigue, infusion-related reactions, and cough in the ofatumumab arm. Grade 3 or higher adverse events occurring more often in the ibrutinib arm included diarrhea (4% versus 2%) and atrial fibrillation (3% versus 0%). Any grade bleeding-related adverse events were more common in the ibrutinib group (44% versus 12%). Additional adverse events more common in the ibrutinib arm included rash (8% versus 4%), pyrexia (24% versus 15%), infection (70% versus 54%), and blurred vision (10% versus 3%). Study treatment discontinuation due to adverse events occurred in 4% of patients in each arm.13
Ibrutinib is an effective therapy for patients with relapsed or refractory CLL/ SLL and patients with deletion 17p. It has been added to the most recent version of the NCCN guidelines as a category 1 recommendation for patients with relapsed or refractory disease.3 Ibrutinib is also being evaluated in untreated patients with CLL or SLL and in various combination therapies in the relapsed/refractory setting.11
Ofatumumab (Arzerra)
Ofatumumab is an IgG kappa human monoclonal antibody that binds to a distinct epitope composed of both small and large loops on the CD20 molecule. Ofatumumab has increased binding and more potent complement-dependent cytotoxicity than rituximab.16,17
Ofatumumab was initially approved by the FDA in October 2009 for the treatment of patients with CLL refractory to fludarabine and alemtuzumab on the basis of durable tumor reduction in a single-arm study. Ofatumumab was administered in eight weekly intravenous in- fusions followed by four monthly infusions with the first dose being 300 mg and doses 2 through 12 being 2,000 mg each. Patients experienced a 42% (99% CI: 26–60) investigator-determined objective response rate (ORR) and 6.5-month (95% CI: 5.8–8.3) median duration of response (DOR). All responses were partial.16,17
Ofatumumab received FDA approval for an additional indication in April 2014. It is now also approved for use in combination with chlorambucil for first-line treatment of CLL in patients for whom fludarabine-based therapy is considered inappropriate,18 based on results presented at the 2013 American Society of Hematology (ASH) Annual Meeting and Exposition.
A multicenter, randomized, open-label study was conducted in 447 patients randomized in a 1:1 manner comparing ofatumumab plus chlorambucil to chlorambucil alone. Patients were considered inappropriate for fludarabine-based therapy due to advanced age and/or comorbidities. Chlorambucil was administered at 10 mg/m2 orally on days 1 through 7 of each 28-day cycle, and ofatumumab was administered intravenously at 300 mg on day 1 and 1,000 mg on day 8 of cycle 1, followed by 1,000 mg on day 1 of subsequent cycles. Patients were treated for a minimum of three cycles, and treatment was continued until best response to a maximum of 12 cycles. Baseline demographics were well matched between treatment arms, with a median age of 69 years, 82% of patients aged 65 years or older, and/or having two or more comorbidities.19
The primary end point of PFS assessed by an independent review committee revealed a significantly longer PFS in the combination-therapy arm compared with the single-agent chlorambucil arm (22.4 versus 13.1 months; HR = 0.57, 95% CI: 0.45–0.73, p < .001). The secondary end point of overall response rate was also improved in the ofatumumab plus chlorambucil arm (82% versus 69%; OR 2.16; p = .001). CR rate was superior in the combination arm compared with the chlorambucil- alone arm: 12% versus 1%, respectively. At a median follow-up time of 29 months, the median OS was not reached for either arm; the trial concluded before survival time could be assessed. The median duration of treatment for both arms was six cycles, with 82% of patients receiving six or more cycles of ofatumumab plus chlorambucil.19
There were similar rates of grade 3 or higher adverse events occurring from the start of treatment through 60 days from the last dose (50% in ofatumumab plus chlorambucil versus 43% chlorambucil alone). The most common grade 3 or higher adverse event occurring in both groups was neutropenia (26% in the combination arm versus 14% in the single- agent arm) followed by infection (15% versus 14%, respectively). Ten percent of patients in the combination-therapy arm experienced grade 3 or higher infusion reactions despite premedication with acetaminophen, an antihistamine, and glucocorticoid; none were fatal.19
Ofatumumab is an important addition to the treatment options for patients with untreated CLL who are not candidates for fludarabine-based therapy. With CLL being diagnosed in older patients with comorbidities, this is an important advance in CLL therapy. Ofatumumab has not been added to the current version of the NCCN guidelines for this setting, but an update is in progress.3
Idelalisib (Zydelig)
Idelalisib is an oral, highly selective PI3K (PI3 kinase) inhibitor approved in July 2014. It is indicated for the treatment of relapsed CLL in combination with rituximab for patients for whom rituximab alone would be considered inappropriate treatment and as monotherapy for patients with relapsed SLL.20
Study 116 was a phase 3, randomized, double-blind, placebo-controlled trial conducted at 90 centers in the United States and Europe comparing idelalisib plus rituximab to placebo plus rituximab in patients with relapsed CLL. Patients were given idelalisib, 150 mg orally twice daily, or placebo with rituximab, 375 mg/m2 followed by 500 mg/m2 every 2 weeks for four doses and then every 4 weeks for three doses (a total of eight infusions). Patients were stratified by presence of 17p deletion or other TP53 mutations or the lack of IgHV mutation. Patients had to have been treated with a CD20 antibody or at least two previous cytotoxic regimens and be ineligible to receive cytotoxic therapy for any of the following reasons: severe neutropenia or thrombocytopenia from previous therapies, creatinine clearance less than 60 mL/min, or CIRS score higher than 6. Patients in the placebo group who experienced disease progression while enrolled in Study 116 were permitted to enroll in Study 117 to receive idelalisib. Patients with progression on idelalisib were allowed a dose increase to 300 mg orally twice daily.21
The groups were well matched with 110 patients randomized to each study arm. The median time on study was short because of early stopping parameters being met due to response. Patients received study treatment for 3.8 months in the idelalisib group and 2.9 months in the placebo group. Results were positive, with the idelalisib combination arm having a significantly improved primary end point of PFS (combination arm, not reached versus placebo arm, 5.5 months; HR = 0.15; p < .001), overall response (81% versus 13%; OR, 29.92; p < .001), and OS at 12 months (92% versus 80%; HR = 0.28; p = .02). Patients receiving idelalisib also experienced lymphocytosis, but this was lessened with the addition of rituximab. Lymphocytosis rates peaked at week 2 and resolved by week 12. Serious adverse-event rates were comparable between groups: 40% in the idelalisib plus rituximab group versus 35% in the placebo plus rituximab group. The most common adverse events in the idelalisib group were pyrexia, fatigue, nausea, chills, and diarrhea.21
Idelalisib’s accelerated approval for relapsed SLL is based on data from a single-arm, phase 2 study (101-09; DELTA) conducted at 41 U.S. and European sites in patients with relapsed indolent lymphoma refractory to rituximab and alkylating-agent containing chemotherapy. Idelalisib was administered at 150 mg orally twice daily until the disease progressed, unacceptable toxicities occurred, or the patient died. A total of 26 patients with SLL were included in this study, and they had an overall response rate of 58% (37%–77%), which was the primary end point. All 15 responses seen in patients with SLL were PRs with a median duration of response of 11.9 months (0–14.7 months). The median duration of treatment was 6.6 months (0.6–23.9 months), and the mean duration was 8.1 ± 5.7 months. The most common adverse events (≥ 20%) seen in all grades included diarrhea, fatigue, nausea, cough, and pyrexia.20,22
Idelalisib is an important addition to the available therapies for CLL/ SLL, having a distinctive mechanism of action. Idelalisib has yet to be added to the NCCN guidelines due to its recent approval and the guideline update currently in progress. It is included in several ongoing clinical trials in combination therapy and untreated patients.3,11,23
Future Directions
There are several new agents that have the potential to provide additional options for the management of CLL. The B-cell lymphoma 2 (Bcl-2) family of regulator proteins is highly involved in apoptosis and is a potential pathway to target in CLL because Bcl-2 is highly expressed in this disease. There are several small-molecule Bcl-2 inhibitors under investigation. The B-cell receptor (BCR) pathway is an additional potential target, as B cells rely on signaling mediated by BCR for maturation, proliferation, survival, and death. Some tyrosine kinases involved in this signaling include spleen tyrosine kinase (SYK), PI3K, and BTK. Inhibitors of these tyrosine kinases are already under investigation.3,11
Conclusion
CLL is a common subtype of NHL and is incurable with current treatment options outside of an allogeneic HSCT. Chemoimmunotherapy has improved OS for patients with CLL, but patients who experience relapsed or refractory disease continue to have poor outcomes. Because of the age of patients at diagnosis, it is important to consider several patient-specific factors and implement appropriate supportive-care measures when selecting a treatment option.3 Identifying treatment alternatives with improved side-effect profiles and patient tolerability is an important next step for management of this malignancy. The recently approved and in-development targeted therapies have the goal of filling this niche.
References
1. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89(11):3909-3918.
2.Altekruse SF, Kosary CL, Krapcho M, et al, eds. SEERCancer StatisticsReview,1975-2007.Bethesda, MD: National Cancer Institute. http://seer.cancer.gov/csr/1975_2007. Based on November 2009 SEER data submission, posted to the SEER website, 2010. Accessed August 11, 2014.
3.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin’s Lymphomas [Version 3.2014]. http://www.nccn.org/professionals/physician_ gls/pdf/nhl.pdf. Published July 18, 2014. Accessed August 11,2014.
4.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA CancerJClin.2014;64(1):9-29.
5.Eichhorst B, Dreyling M, Robak T, Montserrat E, Hallek M; ESMO Guidelines Working Group. Chronic lymphocytic leukemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. AnnOncol.2011;22(suppl 6):vi50-vi54.
6.Gribben JG. How I treat CLL up front. Blood. 2010;115(2):187-197.
7.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: areport from the International Workshop on Chronic LymphocyticLeukemia updating the National Cancer Institute-WorkingGroup 1996 guidelines. Blood.2008;111(12):5446-5456.
8.Robak T. GA-101, a third generation, humanized and glyco- engineered anti-CD20 mAb for the treatment of B-cell lymphoid malignancies. CurrOpinInvestigDrugs.2009;10(6);588-596.
9.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chloarmbucil in patients with CLL and coexisting conditions. N Engl JMed.2014;371(12):1101-1110.
10.US Food and Drug Administration. Drugs: Grazyva (obinutuzumab). http://www.fda.gov/ Drugs/InformationOnDrugs/ApprovedDrugs/ucm373263.htm. Updated November 1, 2013. Accessed August 11, 2014.
11.National Institutes of Health, US Department of Health andHuman Services. ClinicalTrials.gov. http://clinicaltrials.gov/. July16, 2014.
12.Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. NatRevCancer.2014(4);14:219-232.
13.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. NEngl JMed.2014;371(3):213-223.
14.Imbruvica (ibrutinib) receives regular approval by US FDA in chronic lymphocytic leukemia (CLL) and CLL patients with del 17p: approval based on Phase 3 RESONATE data with statistically significant improvements in progression-free andoverall survival. Johnson & Johnson Web site. http://www.jnj.com/news/all/IMBRUVICA-ibrutinib-Receives-Regular-Approval-by-US-FDA-in-Chronic-Lymphocytic-Leukemia-CLL-and-CLL-patients-with-del-17p. Published July 28, 2014. Accessed August11, 2014.
15.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate suboptimal response to therapy. Blood.2014;123(12):1810-1817.
16.Lemery SJ, Zhang J, Rothmann MD, et al. US Food andDrug Administration approval: ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab. ClinCancer Res.2010;16(17):4331-4338.
17.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single- agent CD20 immunotherpay in fludarabine-refractory chronic lymphocytic leukemia.JClinOncol.2010;28(10):1749-1755.
18.US Food and Drug Administration. Ofatumumab. http://www.fda.gov/ Drugs/ InformationOnDrugs/ApprovedDrugs/ucm393823.htm. Updated April 17, 2014. Accessed August 11,2014.
19.Hillmen P, Robak T, Janssens A, et al. Ofatumumab + chlorambucil versus chlorambucil alone in patients with untreated chronic lymphocytic leukemia (CLL): results of the phase III study complement 1 (OMB110911). Paper presented at: American Society of Hematology 55th Annual Meeting and Exhibition; December 7-10, 2013; New Orleans, LA. Abstract 528. https:// ash.confex.com/ash/2013/webprogram/Paper58498.html. Accessed August 11, 2014.
20.US Food and Drug Administration approves Gilead’s Zydelig® (idelalisib) for relapsed chronic lymphocytic leukemia, follicular lymphoma and small lymphocytic lymphoma [news release]. Foster City, CA: Gilead Sciences, Inc. July 23, 2014. http://investors.gilead.com/phoenix.zhtml?c=69964&p=irol-newsArtic le&ID=1950339&highlight=\#sthash.SYMspXkc.dpuf. Accessed August 11, 2014.
21.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. NEngl JMed.2014;370(11):997-1007.
22.Gopal AK, Kahl BS, de Vos S, et al. PI3K inhibition by idelalisib in patients with relapsed indolent lymphoma. NEngl JMed.2014;370(11):1008-1018.
23. US FDA accepts new drug application for Gilead’s idelalisib for the treatment of refractory indolent non-Hodgkin’s lymphoma [news release]. Foster City, CA: Gilead Sciences, Inc. January 13, 2014. http://www.gilead.com/news/press-releases/2014/1/us-fda-accepts-new-drug-application-for-gileads-idelalisib-for-the-treatment-of-refractory-indolent-nonhodgkins-lymphoma. Accessed August 11, 2014.