Launching an Oral Chemotherapy Telehealth Clinic Using the TAMER Model
Diana Tamer, PharmD, BCOP
Clinical Assistant Professor – University of Missouri Kansas City School of Pharmacy, Missouri
Hematology/Oncology Pharmacy Specialist – Advent Health Cancer Center
Shawnee Mission, KS
Background
Oral chemotherapy—also known as oral oncolytics—has been available for nearly seven decades.1 Today, up to 35% of new oncologic agents in the pipeline consist of oral formulations.1 As of December 18, 2020, the Food and Drug Administration (FDA) had 56 treatment approvals for cancer indications; of which 23 were oral chemotherapy.2
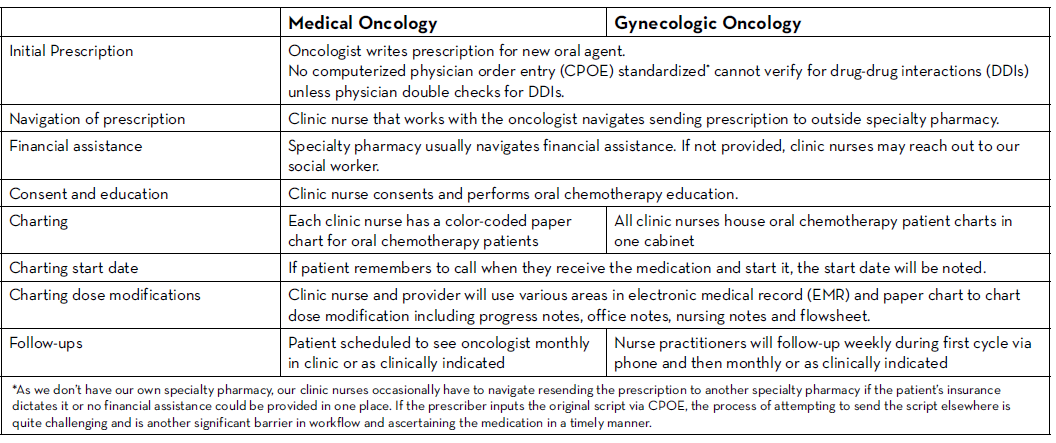
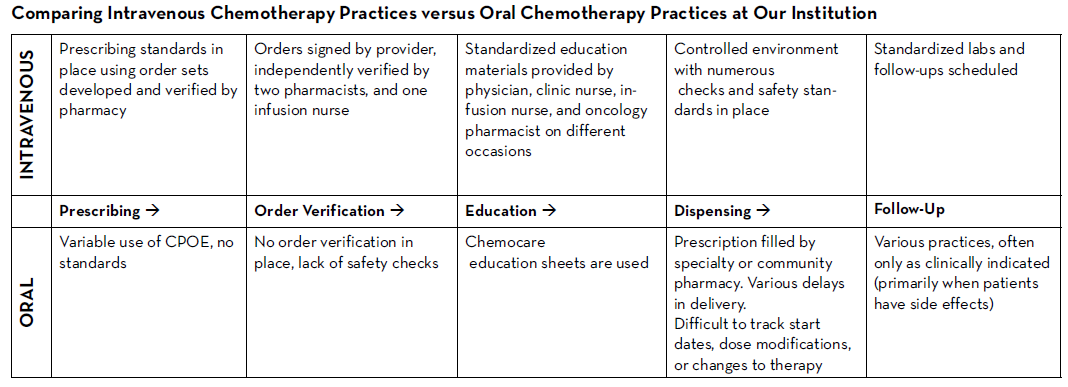
There are many established safety and quality standards for intravenous cancer treatments. Patients treated with intravenous cancer therapies at a cancer center have scheduled visits and are closely monitored. Conversely, national guidelines and workflows for oral cancer therapies vary immensely based on the institution, and continue to be a work in progress. Oral chemotherapy offers cancer patients more flexibility, less disruption to their daily lives, and more autonomy.3 Nevertheless, the effectiveness of these therapies depends greatly on patients’ adherence. Common barriers to patient adherence include complex administration instructions, limited knowledge about the therapy and adverse events, low health literacy, and financial toxicity.4
As these therapies become widely prescribed, it is vital to identify current oral chemotherapy practices at local institutions and learn from successful programs and best practices published by national guiding bodies. Developing a workflow to manage patients on oral chemotherapy requires multidisciplinary collaborative efforts between pharmacists, physicians, advanced practitioners, nurses, social workers, and financial navigators. These efforts require significant staff time to assure patient access to medication, adequate education, proper adherence, and adverse event monitoring, as well as side effect management.
The biggest challenges to implementing oral chemotherapy services are monetary, such as significant non-billable staff time, especially when medications are sent to an external pharmacy and the cancer center receives no compensation in return.5 Successful programs, such as the one by Mancini and colleagues, have profit margins that accommodate a full-time pharmacist, full-time technician, and a full-time pharmacy billing specialist, all dedicated to an oral chemotherapy management program at a community cancer center.6 Oncology pharmacists are the experts on cancer drugs and can play a pivotal role in establishing and managing such programs. Yet, they continue to face financial hurdles of billing due to lack of provider status and the complexity of assigning a dollar value to the interventions and services they provide. Limited studies track the outcomes of pharmacist-led interventions in promoting adherence to, and safety of, oral chemotherapy, especially when oral chemotherapy is decentralized from the cancer center to the community or specialty pharmacies.
Telehealth in Oncology—Teleoncology
One way to overcome the challenges and limited resources for oral chemotherapy is to implement teleoncology, which is the application of telemedicine to advance cancer care, including diagnostics, treatment, and supportive care.7 Disparities in cancer care delivery between intravenous and oral chemotherapy can be improved by establishing telecommunication infrastructure—for example, an oral chemotherapy telehealth clinic, run by clinical faculty contracted with a community cancer center, and staffed by pharmacy trainees.
The World Health Organization (WHO) and the American Telemedicine Association underscore the use of telecommunication to promote health. While telemedicine has gained popularity during COVID-19, lessons learned from this experience may live long beyond the pandemic. Deploying patient-provider telehealth along the oncology care continuum was successfully implemented during the pandemic by a group of providers in California, who highlighted the critical need to further investigate the role of telehealth, not only during crises, but also to improve our routine care of patients with cancer in the future.8
Older Oral Chemotherapy Patient Management at Our Cancer Center
The following outlines an oral chemotherapy telehealth clinic launched by clinical oncology pharmacy faculty and pharmacy student trainees from the University of Missouri Kansas City (UMKC) School of Pharmacy at Advent Health Cancer Center in Shawnee Mission, Kansas.
In an effort to standardize practice across the different clinics and switch from paper charts to a centralized online tracker, the oncology pharmacist had already proposed a new workflow related to the care of patients taking oral oncolytics. The cancer center management team and providers endorsed the proposal and agreed to pilot it; unfortunately, the pandemic delayed the start date. However, various clinical support staff began working remotely, so management suggested that they could partially implement the proposal and switch patients on oral chemotherapy from paper charts to an online tracker.
The oncology pharmacist developed a training video to help navigate the switch from paper charts to the online tracker utilizing the Microsoft Teams platform to include all patients stratified by provider. Data collected in the tracker included the following: patient name, medical record number (MRN), diagnosis, oral oncolytic prescribed, prescription date, dose, insurance provider, specialty pharmacy, patient assistance program, date prescription sent, date prescription received, dates of initial consultation and follow-up with a PharmD, oral chemotherapy initiation date, week 1 through 4 follow-up assessment (by nurse practitioner or PharmD), monthly assessment, telehealth follow-up visit, and notes.
Piloting the New Oral Chemotherapy Remote Patient Monitoring Clinic
Phase I → Switching all patients on oral oncolytics from paper charts to the online tracker
Phase II → Following up on all existing patients via a telehealth visit using the pharmacy consult described below
Phase III → Establishing a standardized workflow for all new patients starting oral oncolytics
The completion of phase I allowed us access to all patients on oral oncolytics in one location. This was followed by developing a pharmacy consult note (including assessment of adherence, adverse events, medication-reconciliation, and a quality-of-life questionnaire) that was approved by providers who wished to enroll patients in this service. We explored multiple telehealth/telemedicine synchronous technologies during the pandemic. These included using the patient portal virtual visits, considering HIPAA protected Zoom, Microsoft Teams, and other mobile applications. Technology challenges included cost, image/sound quality (depending on internet quality), lack of internet and video access for some patients, difficulty remembering scheduled virtual appointments for both patients and providers, patients struggling to use the technology, and the need for more provider training. Phone calls with video capability by patient request worked best for our patients, and hence, was adopted for this telehealth clinic.
Subsequent steps involved training fourth year pharmacy students who were on their advanced pharmacy practice experience (APPE) month-long oncology rotation, and establishing a timeline to cover all patients. The steps below describe the pharmacy students’ training model developed by our faculty.
Student Training—TAMER Model
1. Teach:
- Students learn about the consult note. Each element of the note is reviewed in detail during their first week of rotation.
- Students are assigned patients to work up on Friday.
- Students are instructed to prepare for Monday telehealth visits and set the intention for meaningful and efficient interactions over the phone.
- Students are encouraged to have the patient’s EMR open during the call, as well as multiple databases (drug resource, herbal database, adverse event grading tool) that may be needed.
- Students are provided phone scripts approved by cancer center to introduce the call/telehealth service and to leave messages if patients don't answer.
- Students are instructed to inform faculty immediately in the event a patient discloses any adverse event greater than grade 1.
- Management of grade 1 adverse events such as nausea, diarrhea, constipation, fatigue, and pain are discussed in details and may be addressed during the telehealth visit.
- Students are encouraged to explore cues (unreported adverse events as well as issues identified regarding physical, social, emotional, and functional wellbeing) from the quality of life questionnaire built into the visit.
- Students will complete the consult notes on Monday afternoon. Any interventions are discussed with the faculty and then relayed to the corresponding provider in person on the same day. Faculty will then review all the notes and send edits back to students.
- Edited/reviewed notes are then added to patient’s EMR by the students, co-signed by faculty and forwarded to corresponding provider.
2. Assess Preparedness: The subsequent Monday, the faculty will discuss all the patients that students worked up on Friday and address any questions the students may have prior to any phone calls.
3. Modeling: Faculty models one real patient consult in real time while students shadow.
4. Example: Students then contact their first patient while faculty is present. Faculty may assist during the call if needed, and after completion of the call, faculty will review what went well and what can be improved with the students.
5. Repeat and learn: Students get to participate in this telehealth clinic for three to four Mondays during their rotation.
Challenges
We knew that implementing this new initiative during a global pandemic would be uniquely challenging. Nevertheless, with the collaboration of all cancer team members, we piloted this program with a few providers starting in June 2020. We are currently in the process of retrospectively collecting all the consultation data. This data will be discussed at provider meetings in an effort to get feedback and standardize the management of all patients on oral chemotherapy. A new chemotherapy workflow was developed and scheduled to begin in January 2021. All patients who start oral chemotherapy will be instructed to contact the clinic when they receive their medication to set an initial education/drug-interaction check visit with the oncology pharmacist in person or via telehealth. All patients will have weekly follow-up calls with an oncology pharmacist for the first cycle of therapy; and with a nurse practitioner or clinic nurse for subsequent cycles. Cancer center management and pharmacy will analyze the metrics and explore ways to improve and standardize prescribing and dispensing of these agents.
Closing Remarks
Establishing new, standardized workflows for an oral chemotherapy clinic, in the middle of a pandemic, challenged everyone involved to better observe the needs of their local sites and identify opportunities to innovate with limited resources. Using the TAMER model, our initiative to improve and standardize the oral chemotherapy workflow can lead to improved patient care and outcomes, while also providing a no-cost critical training opportunity for pharmacy students.
Teleoncology is a valuable service that can be utilized by pharmacists to provide access to quality cancer care with minimal disruption to cancer patients. We will continue to identify and measure quality metrics from this new workflow in order to further develop and refine processes to better serve cancer patients.
Acknowledgement
The author acknowledges and thanks Kyla Bidne, PharmD BCPS BCOP and Shanna Keiser, RN, for their guidance and review of the article.
REFERENCES
- DeCardenas R, Helfrich J. Oral therapies and safety issues for oncology practices. Oncol Issues. 2010;(March/April): 40-42. DOI: 10.1080/10463356.2010.11883499
- Food and Drug Administration. Hematology/Oncology (Cancer) Approvals & Safety Notifications. Available online at: https://www.fda.gov/drugs/ resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications. Last accessed December 18, 2020.
- Weingart SN, Brown E, Bach PB, et al. NCCN Task Force Report: Oral chemotherapy. J Natl Compr Canc Netw. 2008;6 Suppl 3:S1-S14.
- Jin J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther Clin Risk Manag. 2008;4(1):269-286. doi:10.2147/tcrm.s1458.
- Mancini R, Kaster M, Vu B, et al. Implementation of a pharmacist managed interdisciplinary oral chemotherapy program in a community cancer center. J Hematol Oncol Pharm. 2011;1(2): 23-30.
- Mancini R, Wilson D. A pharmacist-managed oral chemotherapy program: an economic and clinical opportunity. Oncol Issues. 2012;27(1):28-31.
- Wysocki WM, Komorowski AL, et al. The new dimension of oncology. Teleoncology ante portas. Crit Rev Oncol Hematol. 2005;53: 95–100.
- Liu R, Sundaresan T, Reed ME, Trosman JR, Weldon CB, Kolevska T. Telehealth in Oncology During the COVID-19 Outbreak: Bringing the House Call Back Virtually. JCO Oncol Pract. 2020;16(6):289-293. doi:10.1200/OP.20.00199
