HOPA 2020-2021 Publications Committee
Christan M. Thomas, PharmD BCOP, Editor
Renee McAlister, PharmD BCOP, Associate Editor
Lisa Cordes, PharmD BCOP BCACP, Associate Editor
Andrea Clarke, PharmD BCOP
Jeff Engle, PharmD MS
Ashley E. Glode, PharmD BCOP
Sidney V. Keisner, PharmD BCOP
Chung-Shien Lee, PharmD BCOP BCPS
Heather N. Moore, PharmD BCOP
Gregory Sneed, PharmD
Diana Tamer, PharmD BCOP
Jennifer Zhao, PharmD BCOP
View PDF of HOPA News, Vol. 17, no. 4
Board Update: Anticipating the Green Lights Ahead
David DeRemer, PharmD BCOP FCCP FHOPA
HOPA President (2020–2021)
Clinical Associate Professor, University of Florida College of Pharmacy
Assistant Director, Experimental Therapeutics, University of Florida Health Cancer Center
Gainesville, FL
I ended the last HOPA News Board Update with the message, “much promise lies ahead.” I know I speak for many who look forward to Summer 2021. As you read this, we are in Phase 1 of the COVID-19 vaccination program, which will continue to require significant co-ordination across federal, state, and local authorities.
I am proud of the efforts in our profession to combat COVD-19 and encourage you to read this issue’s cover story, “Oncology Pharmacy and COVID-19: Perspectives from an Early Epicenter” written by Peter Campbell, PharmD, BCOP. It is just a sampling of the ways you all have contributed during this pandemic, and will continue to contribute to immunization, monitoring, and education.
On behalf of everyone on the Board, thank you. You demonstrate compassion and collaboration in the care you provide to the cancer patients you serve. Despite its challenges, 2020 was a successful year, thanks to our resilient volunteers.
2020 Had Many Positives
Advancements in cancer immunotherapy continue to transform our daily practice and the patients we serve. The HOPA Time to Talk Immuno-Oncology™ (TTTIO) toolkit provides patient-focused education on immune checkpoint inhibitors and cellular therapy. I want to recognize the efforts of the TTIO Task Force, especially Chair Heidi Finnes and Vice-Chair Amber Cipriani. Please utilize this tool-kit in your practices; it can be found within the patient education section of our website.
To meet the significant need for online educational programming in 2020, HOPA staff, leadership and volunteers took to the task of converting professional development offerings into virtual content. In December, we held a virtual ASCO Quality Training Program workshop, which was free for members and designed to provide pharmacy professionals with the skills to design and implement quality improvement initiatives. We saw significant interest and received more than 150 applications. We are evaluating another QTP workshop in early 2021, so stay tuned.
Near the end of 2020, we transitioned to a new management company, Executive Director Incorporated (EDI) in Milwaukee, Wisconsin. The Board has been actively working with our new Executive Director, Anne Krolikowski, CAE, to recruit key personnel for team HOPA. We have 18 new team members (of 22 total) providing support and working with HOPA committees. The team is energized and eager to advance our strategic initiatives.
Greenlights in 2021
I recently read Greenlights by actor Matthew McConaughey, but don’t ridicule; it’s currently the #2 hardcover nonfiction on New York Times Best Seller list. It is a complex memoir, with great story-telling you would expect from the actor we have all watched mature. “It’s also a guide to catching more greenlights—and to realizing that the yellows and reds eventually turn green too,” as McConaughey himself describes it.
That statement made me think of our HOPA strategic plan for 2020-2023 and its progression tracker, poignantly covered in red, yellow and green. I know I speak for many in our organization in saying 2020 brought many perceived red lights. But despite our challenges this year, many of our strategic initiatives earned a green designation, which is a testament to our organizational volunteers. I’m confident many more reds and yellows will move to green in the next year.
One 2021 greenlight is the 17th Annual Conference, which will be held virtually. The Board has selected an excellent virtual platform to give attendees an outstanding learning and networking experience. I encourage you to follow marketing announcements for unique engagement opportunities that will be offered prior to and during the conference.
New to this year’s Annual Conference will be the Patient Advocacy Town Hall. It is being planned by the Patient Outreach Committee and it is just one of many efforts within our advocacy strategic pillar this year. I’m excited about the momentum we have going into 2021 and hope you are too.
Oncology Pharmacy and COVID-19: Perspectives from an Early Epicenter
Peter Campbell, PharmD BCOP
Clinical Pharmacy Manager, LeukemiaPGY2 Pharmacy Residency Program Director, Oncology
NewYork-Presbyterian Hospital
Columbia University Irving Medical Center
New York, NY
When SARS-CoV-2 began to snake its way through the boroughs and suburbs of New York City in early 2020, I had been practicing as a board-certified oncology pharmacist and postgraduate year two (PGY2) oncology pharmacy residency program director for several years. I was working mainly in the adult leukemia specialty and was unaware of the impact that the coronavirus was about to have on the city. Like many others, I shrugged off rumors of increasing intensive care unit (ICU) capacity and a new phenomenon called social distancing. Soon enough, I was fully ensnared in a world foreign to my typical daily practice, helping to care for ICU patients suffering from coronavirus disease 2019 (COVID-19).
Big City Becomes Nimble
At the height of the pandemic in April of 2020, New York City was reporting an average of more than 5,000 new SARS-CoV-2 cases per day, with an average of more than 500 deaths per day.1 Many institutions were quickly overwhelmed by this volume of patient cases and the increased demand for emergency room visits and hospital admissions. Many parts of the hospital conventionally used for other purposes (such as conference rooms, lobbies, and waiting areas) were converted to acute care areas.
In a survey of 72 hospitals, it was shown that more than 90% of responding institutions made adaptations to accommodate patients with COVID-19, including the creation of respiratory isolation units.2Likewise, personnel were redeployed and repurposed to help care for a massive influx of acutely ill patients. With my inpatient leukemia service dwindling, my colleagues and I found ourselves volunteering to provide clinical pharmacy services for the rapidly expanding intensive care unit (ICU) patients. This redeployment of personnel extended greatly beyond pharmacy, with providers of all disciplines being used to fill sometimes novel roles to optimize the care model.3
Residents Learn on the Frontline
While my normal days previously consisted of reviewing chemo-therapy regimens and providing clinical care to oncology patients, I was now reviewing sedative and vasopressor drips and refreshing my knowledge by reviewing critical care guidelines and standard operating procedures. As a residency program director, I developed ways for the residents to be involved in the care of these complex patients, which proved to be both a challenge and an opportunity. Out of necessity, the resident’s learning experiences were augmented in order to juggle the needs of both the institution and their residency requirements. We developed schedules and workflows that allowed residents to assist in clinical care and sterile compounding, while also making sure that no required learning experience was neglected or forgone.4
Patient Volume Outpaces Drug Inventories
To further complicate care for COVID-19 patients, there was an onslaught of drug shortages. Some that impacted us the most were intravenous sedatives and analgesics.5 Due to the increased number of intubated patients, many pre-mixed sedatives and analgesics became difficult to acquire, forcing hospitals to either admix these agents or switch patients to therapeutic alternatives when possible. The admixture of these agents necessitated a vast shift in staffing resources, as the volume substantially exceeded our normal operations.
In an effort to better manage our drug inventory, processes were also established to allocate agents on shortage to specific patient populations or specified patient care units. Twice weekly meetings were held to ensure all stakeholders were knowledgeable of current inventory levels, to disseminate drug bulletins, and discuss optimal patient care strategies.
An Influx of New Literature
The increasing volume of new literature also posed a challenge. During the height of the COVID-19 pandemic, new literature was being published at a frenzied pace. It has been estimated that over 23,000 unique documents relating to COVID-19 have been published in 2020 alone. While these documents include letters, editorials, and review articles, nearly 50% are original research.6 My colleagues and I were responsible for reviewing and interpreting the ever-changing body of literature and resulting clinical management of this patient population. This literature was not limited just to therapeutics targeting COVID-19, but also to supportive care such as anti-inflammatories and venous thromboembolism prophylaxis and treatment.
With such poor outcomes in such a high volume of patients, many providers were desperate to find any therapy that may be beneficial for suffering patients. This desperation proved to be a double-edged sword, as clinical decisions were often being weighed before fully knowing the potential toxicities or implications of using these therapies.
Hydroxychloroquine proved to be the perfect case study in this situation; widespread use of it quickly dissipated as its benefit among hospitalized patients dwindled.7 As the flurry of literature continued to prompt questions regarding new therapies and clinical practices, my colleagues and I met twice weekly to discuss the merits and disadvantages, as well as to share anecdotes and experiences. This was in addition to the daily communication occurring amongst smaller groups with more direct knowledge and experience using certain therapeutics. As an oncology clinical pharmacist, I leaned heavily on the experience and expertise of my critical care and infectious disease clinical pharmacist colleagues to better care for these patients. I also contributed my oncology pharmacy knowledge to the debate by routinely discussing the pharmacotherapy of agents such as tocilizumab with my infectious disease colleagues, who had less experience using these agents.
Ultimately, a New Normal
As the rates of new infections and deaths began to fall throughout New York City, our normal clinical duties resumed. While the pandemic spread and ravaged other parts of the United States, a new normal was established, a normal in which vigilance and caution reins the day. Eventually, patient volumes returned to pre-pandemic levels and we all returned to caring for patients within our own specialties but we won't forget the lessons learned and experiences gained during a fateful, and now infamous, 2020.
REFERENCES
- New York City Department of Health and Mental Hygiene. COVID-19 data. https://www1.nyc.gov/site/doh/covid/covid-19-data.page. Accessed September 25, 2020.
- Auerbach A, O’Leary KJ, Greysen SR, et al. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020 August; 15(8):483-488.
- Kumaraiah D, Yip N, Ivascu N, Hill L. Innovative ICU Physician Care Models: Covid-19 Pandemic at NewYork-Presbyterian. NEJM: Catalyst. 2020. Accessed May 5, 2020.
- Campbell P, Witenko C, Dzierba AL. Perseverance in a pandemic: a unique pharmacy residency learning experience. Am J Health-Syst Pharm.
From Skeptic to Believer: How an oncology pharmacist, mom, and recovering workaholic learned to embrace integrative medicine
Jill S. Bates, PharmD MS BCOP FASHP RYT-200
Associate Professor of Clinical Education
University of North Carolina Eshelman School of Pharmacy
Chapel Hill, NC
PHASER Pharmacy Program Manager
Department of Veterans Affairs
Durham, NC
During an overwhelming season, my entire family came down with influenza. When I got sick, I hadn’t even recovered from an earlier upper respiratory infection. I suffered classic symptoms of influenza; except, non-classically, I continued to cough after my fever, aches, and chills subsided.
I coughed and coughed. I was not able to breathe. As an avid runner, as you can imagine, this was problematic for me. If I could not run, I could not keep my body and mind healthy. I slowly unraveled and this leaked out into the physical realm. Insomnia. Digestive issues. Injuries. Worst of all, respiratory issues. I couldn't breathe, and truly, when you can't breathe, nothing else really matters. I felt desperate.
I was referred to a pulmonologist after multiple months without getting better and I was diagnosed with reactive airway disease from influenza, and eventually, asthma. I started medications to control my symptoms; at one point, I was on five when previously I was on none. Initially, the medication helped me breathe, but over time, I felt worse with each dose. I discussed this with my doctor.
A Resilient Mindset
I was not improving like I thought I should, I explained. I am not sure I am on the right medication, I reasoned. I did not understand biologically what was happening with my body. My doctor replied, “I think you are a woman who has been relatively healthy your whole life. Now you are not as healthy as you once were and you cannot handle it.” Gut punch. These were painful words, especially as a healthcare provider myself. It seemed the system that I worked within was not supporting me. Feeling as though I was out of options, I decided to try integrative healthcare approaches.
I was reading the book “The Body Keeps the Score” by Bessel van der Kolk. A clinical psychologist, Van der Kolk believes that trauma is residue from the past as it settles into your body. “When people are traumatized, they become afraid of their physical sensations, their breathing becomes shallow, and they become uptight and frightened about what they’re feeling on the inside. Yoga opens you up to feeling every aspect of your body’s sensations. It’s a gentle, safe way for people to befriend their bodies, where the trauma of the past is stored.”1 He believes trauma is a somatic issue; it’s in your body. This is what brought me to yoga.
Former Dancer Drawn to Yoga
A dancer in my youth, I was immediately drawn to yoga postures. As a runner, I also needed a complementary athletic plan to prevent injury by building strength and re-gaining lost flexibility. I did not heal effortlessly or overnight. My journey to health took a tremendous amount of time and effort. But I owned my story and it taught me that my health is multidimensional. I know my body best and it is no one else’s job to take care of me, but me. I do have control over my body, mind, and spirit, and I need to slow down so I can listen to what all of me has to say. Integrative healthcare practices, like yoga, are not unfounded and can support overall well-being. Personally, practicing yoga gives me the ability to be still and connect with God; with Jesus, my ultimate source of rest.
As a professional, full-time working mom of two young children, my life is busy just like yours. Unfortunately, I have all the tendencies that can lead to a frenzied busy state: Overwhelm, compassion fatigue, burnout, and ultimately, illness. Maybe you can relate? Yoga helps me let that go and grow in quiet strength. I started Chill Pill Yoga (CPY) to share the practice of yoga with busy professionals, like pharmacists. These days we hear so many suggestions to breathe, engage in yoga, be mindful or meditate, but how does one do that? I hope to share content that provides a playbook. CPY is new and I hope this community grows over time.
On the yoga mat, I find a place of peace, of wholeness. My story led to a concession that rest is an essential component of sustain-able self-care; it is needed to create white space for the soul. On the yoga mat, I can leave all my type A tendencies behind and be still. Connecting with my practice off the mat, I made several changes to my professional lifestyle. I engaged in an iterative process of evaluating how I spent my time and whether this reflected my values. I made changes where there were discrepancies. I created honest boundaries that honor my limits, and I made a conscious decision to focus on the process, not results. When it comes to compassion fatigue, I strive to live the adage “the best defense is a good offense.”2
Connect with Chill Pill Yoga
Please visit chillpillyoga.com. From there, you can sign up for the newsletter, take a live-stream class, or start following CPY on social media.
REFERENCES
- Kripalu center for yoga and health. https://kripalu.org/resources/how-yoga-helps-heal-trauma-qa-bessel-van-der-kolk [accessed 9/30/2020].
- Compassion fatigue project. https://www.compassionfatigue.org/TheTenLawsHealthyCaregiving.pdf [accessed 9/30/2020].
Chair Time Optimization
Kristin A. Hutchinson, PharmD
Oncology Clinical Pharmacist Trellis Rx
Atlanta, GA
Katelyn M. Brown PharmD Candidate 2021
University of Mississippi School of Pharmacy
Jackson, MS
Gregory T. Sneed, PharmD
Assistant Professor, Clinical Pharmacy and Translational Science
University of Tennessee Health Science Center College of Pharmacy
Memphis, TN
Alexander R. Quesenberry, PharmD BCOP
Pharmacy Director Baptist Cancer Center
Memphis, TN
Introduction
Like many institutions nationwide, Baptist Cancer Center (BCC) has grown since its inception to include several infusion centers and remote clinics. One of the challenges of multiple sites spread throughout greater Memphis, is standardizing practices so that patients can have consistent, excellent care regardless of the treatment location.
One variation we discovered that could potentially translate into improved patient satisfaction, and eventually increased revenue, involved our premedication process. At many of our treatment centers, the medications meant to prevent reactions and adverse effects from chemotherapy were taking nearly as long to prepare, administer, and dwell as the chemotherapy administration itself. By streamlining as much of this process as possible, the improved efficiency would result in a lighter workload for the pharmacy department and nursing staff, and shorter infusion time for patients.
Three-Part Pilot Program
Over the course of several months, BCC’s busiest infusion center piloted process improvements using, in part, the National Comprehensive Cancer Network (NCCN) workgroup efficiency study as a model. The patients we targeted to measure these changes were those patients receiving carboplatin chemotherapy and 5 premedication agents.
The first modification we implemented to our premedication process began at the pharmacy level and involved moving from mixing IV piggyback mini-bags to providing nursing staff with IV push medications when possible. Shortly thereafter, medications that were available in oral formulations became preferred when appropriate for therapy.
Once oral and IV push formulations were adopted, the second major improvement was installing and integrating an automated dispensing machine for nursing staff to have immediate access to premedications upon order release and pharmacist verification.
The next and most recent improvement was to educate infusion nursing staff on how to maximize efficiency. Training included administering agents not requiring antiemetic treatment or hyper-sensitivity prophylaxis during premedication dwell time. Our pharmacists compiled a list of antineoplastic medications that would be appropriate to administer without premedication, and that were frequently used alongside our carboplatin-containing regimens. This list included medications such as bevacizumab, pembrolizumab, and trastuzumab, among others. When patients meeting inclusion criteria also received one of these agents, infusion nurses were encouraged to hang these agents during the 30-minute window that had previously been utilized only to allow antiemetics and antihistamines to be absorbed and effective.
So Far, Improved Efficiency and Less Chair Time
These changes have resulted in improved efficiency for staff and less time in infusion chairs for patients. In fact, recent data from our infusion center shows that our patients meeting inclusion criteria are spending an average of 42 minutes less per treatment in our infusion center chairs than they were at baseline, not quite a year and a half ago. Over time, we expect this multi-pronged approach to continually improve and extend far beyond this subset of patients. By manipulating variable factors where best practices do not yet exist, we can give patients back a bit of their day while still ensuring adequate antiemetic treatment and hypersensitivity prophylaxis. Optimizing patient flow through the infusion center over time will allow for more patients to be treated with minimal additional resources, improving revenue in the long term.
Patient Satisfaction
Patient satisfaction has become a major focus nationwide following mandates of the Affordable Care Act and its Centers for Medicaid and Medicare (CMS) reimbursement implications.2 Research has shown that patient satisfaction can be tied to patient perception of quality of healthcare and ultimately in clinical outcomes.3 Patient satisfaction has always been a priority at BCC, but improvements can and should always be made. Not surprisingly, we have found from previous patient satisfaction surveys, that patients value their time. We have extended hours at one of our infusion centers to allow patients flexibility, and started using clinically appropriate faster infusion rates in certain chemotherapy regimens to minimize the time our patients spend in the clinic. There is evidence that implementing a series of changes over time improves outcomes more effectively than maintaining a single alteration.4,5
In keeping with that philosophy, the BCC infusion department implemented our step-wise quality improvement initiative to optimize chair time, which we hope will demonstrate our continued commitment to our patients while improving our own workflow.
For Patients, Quality Care Linked to Wait Times
Delivering efficient care is important for a number of reasons. Patient satisfaction correlates with reduced waiting time.2,3,6 Patients’ own perceptions of quality of healthcare, in fact, correlates with waiting time.3 Satisfaction is crucial to our continued development as a cancer center, and it ultimately can influence reimbursement rates and patient clinical outcomes. Adopting more efficient procedures will reduce chair time, potentially increasing patient turnover and revenue long term.
According to an NCCN study, data at one institution indicated that an infusion center chair is associated with $730 direct margin per hour.1 A study from MD Anderson Cancer Center in 2010, showed that implementing efficiency strategies “translated into more than $1 million in annualized potential financial opportunity for the cancer center.”1,4
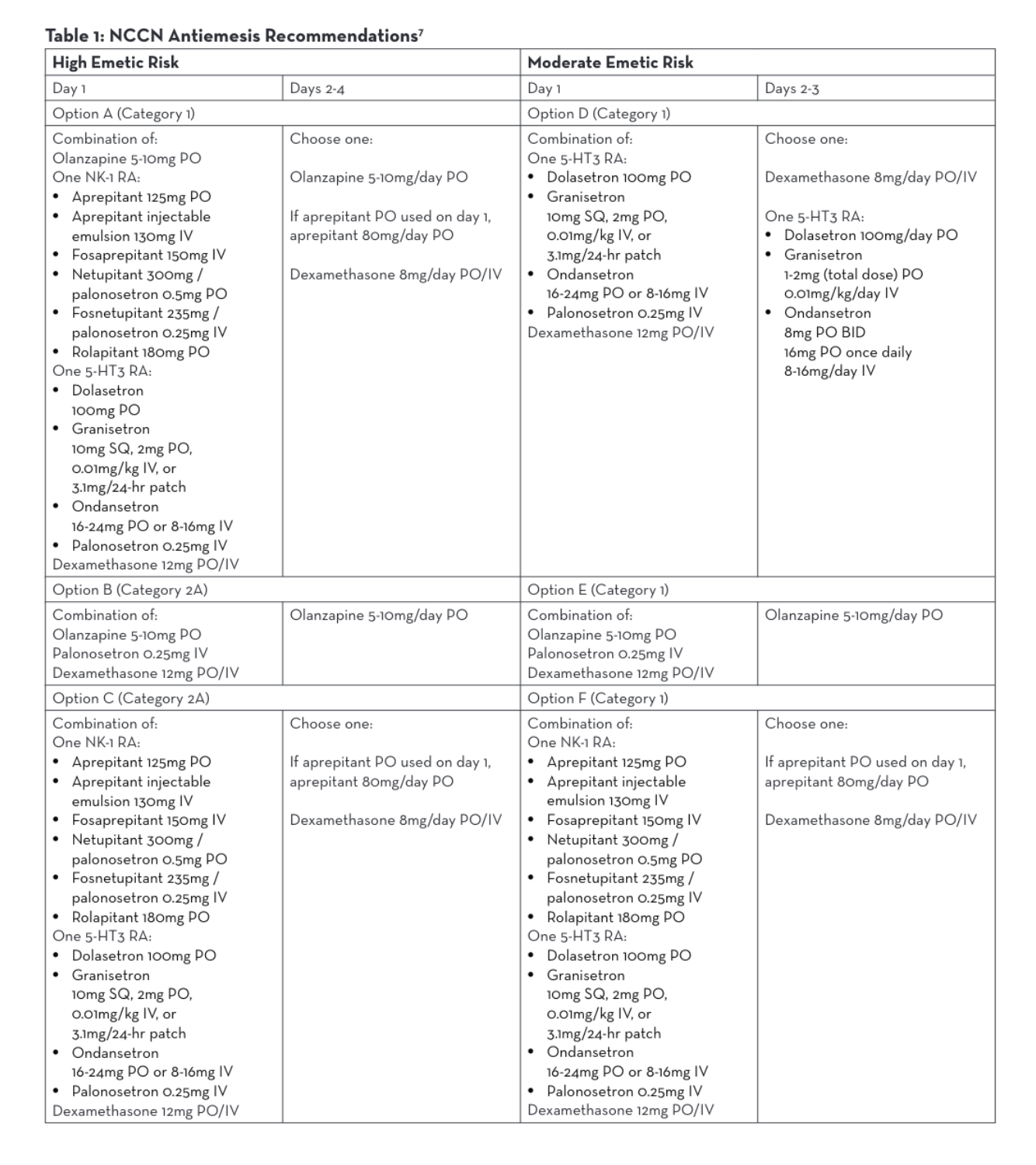
Agents used for HEC (Highly Emetogenic Chemotherapy)/MEC (Moderately Emetogenic Chemotherapy)
Although hypersensitivity reaction prevention and antiemetic regimens are well studied, best practices do not yet exist for efficient premedication administration. The NCCN has recently begun surveying cancer care institutions nationwide in order to develop efficient and effective premedication processes, but currently the routes of administration, preparations of these medications, and dwell time of these medications once administered vary widely.1
Each of the 18 centers who responded to the NCCN survey reported a different premedication regimen for the same highly emetic chemotherapy treatment.1 Two centers that reported no wait time administered 3 oral medications concurrently—aprepitant, dexamethasone, and either ondansetron or granisetron.1 The center with the longest wait time of 60 minutes reported giving fosaprepitant IV individually followed by dexamethasone IV and palonosetron IV given concurrently.1
The NCCN Antiemesis Guidelines consistently allow for intravenous or oral formulations within 6 different combination options of antiemetic medications for parenteral chemotherapy, regardless of the emetic risk.7 Antiemesis regimens may consist of olanzapine, a neurokinin-1 receptor antagonist (NK-1 RA), a serotonin receptor antagonist (5-HT3 RA), or dexamethasone. Both oral and intravenous NK-1 RAs and 5-HT3 RAs may be utilized per NCCN (Table 1).7 Many studies have been done to demonstrate similar efficacy between oral and intravenous antiemetic medications and neither NCCN nor Multinational Associate of Supportive Care in Cancer (MASCC)/European Society of Medical Oncology (ESMO) guidelines address a preference.7,8,9,10,11 Therefore, BCC administers antiemetic regimens that are both efficacious and also lessen chair time.
One aim of Baptist Cancer Center’s Chair Time Optimization Project tested the adoption of oral and IV push premedication strategies. More specifically, when appropriate and available as an oral formulation, we use oral premedication. Examples include dexamethasone PO and ondansetron (Zofran®) PO. If medications are available in an IV-only formulation or a chemotherapy regimen specifies IV administration of premedication, we prefer a push over a piggyback barring anticipated adverse effects. By implementing these protocols, we have lessened chair time for select patients.
Antiemetic Agent Approval and Dosage Form Advancements
The manufacturers of antiemetic agents used within MEC and HEC have made advancements that assist in chair time optimization. A variety of changes to prescribing information based on clinical studies has allowed for more rapid administration of antiemetic agents compared to their original approval. In November 2017, aprepitant (Cinvanti) was originally approved as an intravenous infusion over a period of 30 minutes. In February 2019, the prescribing information was updated to include the approval of intravenous injections over a period of 2 minutes.12 The prescribing information for the majority of agents does include that the injection or infusion should be completed approximately 30 minutes prior to chemotherapy; however, studies have shown that the use of these agents within as quickly as 5 minutes before administration of chemotherapy demonstrated comparable antiemetic safety and efficacy.13 In addition, the available dosage forms of antiemetic agents have changed as well. The majority of agents are now available as an oral tablet, reconstituted solution, or even a prefilled syringe. The availability of these new dosage forms allows for stocking of the medications in automated dispensing cabinets for easier nursing access and expedited delivery to the patient.
New 797 Standards and Challenges in the Clean Room
Chemotherapy premedication route strategies must take into consideration new USP (US Pharmacopeia) 797/800 compounding standards. Many oncology outpatient IV room setups may be limited in meeting the environmental requirements to allow for the longest available beyond use dating. This limited beyond use dating (12 hours) can restrict the use of batch preparations and may cause additional demand for IV premedication mixing during peak rush hours. Forgoing the requirements of mixing IV premedications within an IV piggyback can allow pharmacy staff to focus their efforts on chemo-therapy preparations and improve compounding time.
It is important to note a few additional steps that will impact oncology pharmacy practices; including new Drug Supply Chain Security Act (DSCSA) requirements of recording of lot numbers on compounded preparations and USP 800 implications that require all products, including premeds made in the biologic safety cabinet, or BSC, where hazardous products are compounded to require “PPE precaution handling required.”14
Costs and Impact on Reimbursement
When discussing changes in the route of administration one must consider the potential financial gain and loss. Compounding IV medications requires an infusion bag, IV tubing, syringes and needles. These supplies, as well as the labor of pharmacists and technicians, have a direct cost. By administering these agents by an IV push or via an oral route, infusion centers can avoid or reduce those direct costs. However, due to outpatient billing, potential exists for lower reimbursement in nursing administration fees. Nursing medication administration activities are generally reimbursable in the outpatient setting for IV infusions (96367) and for IV push (96375).16 2020 Medicare payment rates for those nursing administration codes are estimated at $31.40 and $16.60 respectively.16
It is also worth noting that Medicare part B does not individually pay for medications that fall under the packaging threshold ($130 as of 2020).17 Ideally, if the medication falls under the packaging threshold, the nursing IV push or infusion administration fees should cover the potential expense of the medication plus direct costs. Therefore, it might be most financially advantageous to utilize an inexpensive generic oral premedication.
Summary
At Baptist Cancer Center, variability in our premedication administration practices resulted in inefficiency for our staff and inconsistency for our patients. Since best practices do not yet exist, we found flexibility to establish our own. Dosage form advancements by manufacturers have provided faster yet equally efficacious administration. Utilizing oral formulations where available may minimize cost. By encouraging oral and intravenous push administration where appropriate for antiemetic treatment and hyper-sensitivity prophylaxis, infusion centers that might otherwise have been challenged by USP 797/800 constraints will have the ability to adequately premedicate patients for chemotherapy. We expect over time that patient satisfaction will continually improve, which is critical to reimbursement, and ultimately, patient outcomes. By implementing changes favoring efficiency and optimizing patient chair time, infusion centers and patients both can benefit.
REFERENCES
- Sugalski J, Kubal T, Mulkerin D, et al. National comprehensive cancer network infusion efficiency workgroup study: optimizing patient flow in infusion centers. J Oncol Pract. 2019; (15)5: e458-e466.
- Gourdji I, McVey L, Louiselle C. Patients’ satisfaction and importance ratings of quality in an outpatient oncology center. J Nurs Care Qual 2003; 18: 43-55.
- Sandoval GA, Brown AD, Sullivan T, Green E. Factors that influence cancer patients’ overall perceptions of the quality of care. Int J Qual Health Care. 2006; 18: 266-274.
- Kallen, M. A., Terrell, J. A., Lewis-Patterson, P., & Hwang, J. P. Improving wait time for chemotherapy in an outpatient clinic at a comprehensive cancer center. J Oncol Pract; 2012; 8(1), e1–e7.
- Santibanez P, Chow VS, French J, et al. Reducing patient wait times and improving resource utilization at British Columbia Cancer Agency’s ambulatory care unit through simulation. Health Care Manag Sci 2009; 12: 392-407.
- Gesell SB, Gregory N. Identifying priority actions for improving patient satisfaction with outpatient cancer care. J Nurs Care Qual. 2004: 19: 266-233.
- Ettinger D, Berger M, Barbour S, et al. National comprehensive cancer network clinical practice guidelines in oncology: antiemesis. NCCN. 2020;2:9-13.
- Aapro M, Gralla R, Herrstedt J, et al. Multinational association of supportive care in cancer / european society for medical oncology antiemetic guideline 2016 with updates in 2019. MASCC/ESMO. 2019.
- Gandara, D. R., Roila, F., Warr, D., Edelman, M. J., Perez, E. A., & Gralla, R. J. Consensus proposal for 5HT3 antagonists in the prevention of acute emesis related to highly emetogenic chemotherapy. Dose, schedule, and route of administration. Supportive Care in Cancer. 1998; 6(3), 237-243.
- Spector JI, Lester EP, Chevlen EM, et al. A comparison of oral ondansetron and intravenous granisetron for the prevention of nausea and emesis association with cisplatin-based chemotherapy. Oncologist 1998; 3: 432-438.
- Jordan K, Sippel C, Schmoll HJ. Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: past, present, and future recommendations. Oncologist 2007; 12: 1143-1150.
- Drugs@FDA: FDA-Approved Drugs. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
- Perez EA, Lembersky B, Kaywin P, Kalman L, Yocom K, Friedman C. Comparable safety and antiemetic efficacy of a brief (30-second bolus) intravenous granisetron infusion and a standard (15-minute) intravenous ondansetron infusion in breast cancer patients receiving moderately emetogenic chemotherapy. Cancer J Sci Am. 1998;4(1):52-8.
- The drug supply chain security act: improving the integrity of drug distribution. POWER-PAK CE. https://www.powerpak.com/course/content/113032.
- USP general chapter hazardous drugs-handling in healthcare settings. USP. https://www.usp.org/compounding/general-chapter-hazardous-drugs-handling-healthcare.
- Final 2020 Medicare coding & payment for drug administration services under the physician fee schedule. American Medical Association. 2019.
- Atkins E, Trunk S. 2020 proposal for Medicare Part B drug reimbursement: Business as usual. JD Supra. 2019.
Pharmacy Telehealth Services — Efficient and Safe Quality Care Before and During the COVID-19 Pandemic
Michelle K. Azar, Doctor of Pharmacy Candidate 2021
University of Michigan College of Pharmacy
Ann Arbor, MI
Introduction
Telehealth is the use of electronic information and telecommunication technologies to provide long-distance health care and education to patients.1 The use of technology within the healthcare system has become a fundamental part of providing safe and effective patient care.2 Telehealth services increase access to healthcare, enhance coordination of care, decrease the burden of travel, reduce the overall cost of care, and bring specialized individuals into areas that initially lack access to them.3
The use of telehealth services has become even more critical with the COVID-19 pandemic and the need to provide quality care that keeps both providers and patients safe. Within a short period of time, many pharma-cy services have transitioned to telemedicine in order to meet patient care needs while maintaining a safe environment. While outcomes of many remote pharmacy services are not published, several publications demonstrate the effective implementation of telehealth services to provide exceptional patient care.
Pharmacy Services within Primary Care
Approximately 33% of military veterans live in rural areas that lack access to specialty care providers. Employing telehealth technology to provide pharmacy services can increase access and improve outcomes for this patient population. The Northwest Regional Virtual Integrated Multisite Patient Aligned Care Team (V-IMPACT) Hub stationed in Boise, Idaho, is a multicenter program that reaches remote locations across the United States.3
A study of the remote clinical pharmacy services in this program included 544 unique patients and 3,400 visits where encounters were conducted via clinical video telehealth (CVT) or telephone from October 2014 to March 2017. In the diabetes group, 242 patients were seen by a pharmacist, and the mean absolute reduction from baseline in HbA1c values was 1.61%. Fifty-five percent (132/242) of patients were discharged at goal. At discharge, 59 patients (42%) had achieved tobacco cessation, and 55 (39%) had achieved a reduction in tobacco use but not complete cessation. These results suggest that pharmacists providing primary care comprehensive medication management services via telehealth improved disease management and was an effective tool for providing patient care.
Pharmacy Services within Anticoagulation Clinics
Clinical pharmacists at a VA medical center implemented telehealth services to provide anticoagulation therapy management services to patients off-site.4 The clinical pharmacy specialist provided direct patient care, guided the telehealth technician in performing physical assessments when necessary, conducted interviews, evaluated the patient’s warfarin therapy, and formulated a therapeutic plan.
The impact of the use of video technology on patients’ INR values and patient satisfaction was evaluated; the mean percentage of time patients’ INR values were within the therapeutic range and remained stable (about 81%, compared with about 77% under the previous in-person model). Implementation of remote anticoagulation monitoring services enabled pharmacist resources to be reallocated to other duties and expanded access to healthcare in rural areas while maintaining positive patient outcomes and satisfaction.
Remote Pharmacy Services during the COVID-19 Pandemic
Healthcare systems were pressed to develop innovative ways to provide high-quality patient care that were both safe and effective within a short period of time as the COVID-19 pandemic unfolded. The University of Washington (UW) Medicine was one example of a medical center that altered their delivery of clinical services.
In early March, the Centers for Medicare and Medicaid modified its regulations to expand pharmacists’ ability to provide telehealth services. UW credentialed and trained pharmacists to provide comprehensive medication management via telehealth to patients in primary care and specialty clinics. From March 31 through April 28, 2020, clinical pharmacist telehealth services including anticoagulation, pain management, primary care, oncology, and other specialty areas were offered to 139 patients of which 83% (n = 116) completed these visits.5 These visits offered significant advantages during the pandemic, including flexibility in scheduling appointments, decreased burden of traveling, personalized communication, increased caregiver participation, the ability to visually review the patient’s medications or injection technique remotely, and avoidance of office space limitations for in-clinic visits. While outcomes and metrics are needed to evaluate the impact of this transition on patient care, telehealth services created an avenue for meeting patient needs.
Oncology Practice during the COVID-19 Pandemic
According to Al-Shamsi et al., patients with cancer have an estimated two-fold increased risk of contracting COVID-19 than the general population.6 One method to decrease risk is to use telemedicine to minimize face-to-face visits which can help mitigate exposure and further transmission. Examples of successful telemedicine in oncology include remote chemotherapy supervision and education, symptom management, survivorship care, palliative care, and clinical trials.
The University of Rochester Specialized Oncology Care and Re-search in the Elderly (UR SOCARE) clinic, an interdisciplinary care team that receives referrals from oncologists, was able to switch to telehealth services during the pandemic.7 As part of the care team, a pharmacist meets with a patient via telephone for medication re-view and to identify potential interventions. This system provided elderly patients with a safe alternative for oncology care without putting them at risk for exposure to COVID-19.
A team at Memorial Sloan Kettering Cancer Center (MSK) created a program to detect patients who tested positive for the virus and a protocol for providing at-home care.8 Each day patients completed a 10-question electronic or telephone survey to report any COVID-19 symptoms. Based on severity of symptoms, an automated alert was sent to the care team, which would determine follow-up.
Between March 26 and June 17, 2020, the team enrolled 763 patients who filled out 10,044 questionnaires. Of the 239 patients who completed the satisfaction survey, 92% felt the time and effort to report symptoms was worth it, 93% of those with a pulse oximeter agreed that it made them feel more comfortable being at home, 90% felt connected and safe with the COVID-19 management team, and 62% felt that taking part in the program helped prevent visits to the emergency room or urgent care center. This program allowed successful monitoring of cancer patients diagnosed with COVID-19 while keeping healthcare providers and other patients safe from potential infection.
Conclusion
Telemedicine services have expanded rapidly in recent years, with the COVID-19 pandemic drastically accelerating this process. Several studies have demonstrated the benefit of utilizing telemedicine for providing safe and effective patient care in various settings, however, further studies are needed to demonstrate the wide-ranging benefit to patients with cancer. In addition, more studies are needed to measure specific outcomes and metrics for programs implemented. Oncology pharmacists are in a prime position to continue to cultivate and utilize telehealth services to provide high-quality patient care while demonstrating outcomes.
- Badowski ME, Walker S, Bacchus S, et al. Providing Comprehensive Medication Management in Telehealth. Pharmacotherapy. 2018;38(2):e7-e16. doi:10.1002/phar.2071
- Wechkunanukul K, Parajuli DR, Hamiduzzaman M. Utilising digital health to improve medication-related quality of care for hypertensive patients: An integrative literature review. World J Clin Cases. 2020;8(11):2266-2279. doi:10.12998/wjcc.v8.i11.2266
- Litke J, Spoutz L, Ahlstrom D, et al. Impact of the clinical pharmacy specialist in telehealth primary care. Am J Health Syst Pharm. 2018;75(13):982-986. doi:10.2146/ajhp170633
- Singh LG, Accursi M, Korch Black K. Implementation and outcomes of a pharmacist-managed clinical video telehealth anticoagulation clinic. Am J Health Syst Pharm. 2015;72(1):70-73. doi:10.2146/ajhp130750
- Segal E, Alwan E, Pitney C, et al. Establishing clinical pharmacist telehealth services during the COVID-19 pandemic, Am J Health Syst Pharm. 2020; 77(17): 1403–08. doi.org/10.1093/ajhp/zxaa184
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25(6):e936-e945. doi:10.1634/theoncologist.2020-0213
- DiGiovanni G, Mousaw K, Lloyd T, et al. Development of a telehealth geriatric assessment model in response to the COVID-19 pandemic. J Geriatr Oncol. 2020;11(5):761-763. doi:10.1016/j.jgo.2020.04.007
- Majeed J, Garcia J, Holland J, et al. When a cancer patient tests positive for Covid-19.” Harvard Business Review, July 16, 2020. hbr.org/2020/07/when-a-cancer-patient-tests-positive-for-covid-19. Accessed September 20, 2020.
Chemotherapy and Immune Checkpoint Inhibitor Combination Regimens: How do we manage corticosteroid use to prevent adverse effects from chemotherapy?
Sara Moran Smith, PharmD BCOP
Hematology/Oncology Clinical Pharmacist
M Health Fairview
Minneapolis, MN
Up until about three years ago, it seemed counterintuitive to combine immune checkpoint inhibitors (ICI) and chemotherapy, given the immunosuppressive properties of chemotherapy and the theoretical potential to decrease the efficacy of ICI. Today, we have a number of FDA-approved regimens that combine ICI and chemotherapy in the front-line setting. Major advances were made in lung cancer with multiple regimens approved for metastatic non-small-cell lung cancer and extensive-stage small-cell lung cancer.1,2 In addition, pembrolizumab was recently studied in combination with chemotherapy for early stage triple-negative breast cancer in previously untreated patients.3
With the use of ICI, medication management question arise. Specifically, how are corticosteroids best managed to ensure minimal impact on efficacy while preventing adverse effects of chemotherapy, such as nausea, vomiting, skin rash, and hypersensitivity reactions? Corticosteroids have long been known for their immunosuppressive properties; however, their anti-inflammatory action is quite complex and not fully understood. Corticosteroids suppress effector T-cells and increase regulatory T-cells, which results in decreased inflammation, immune activity, lymphopenia and impaired T-cell response to antigen. Corticosteroids likely bring a balance between costimulatory and coinhibitory signals rather than overall direct suppression of the immune system.4,5 The effects on the immune system from the con-current administration of corticosteroids and ICI remain unknown.
Data in Non-Small-Cell Lung Cancer
The trials supporting front-line use of chemo-immunotherapy maintained corticosteroid use for prevention of nausea and vomiting or skin rash.
In the IMpower130 trial, metastatic non-small-cell lung cancer patients were randomized to receive nab-paclitaxel and platinum agents plus or minus atezolizumab. Nab-paclitaxel is considered to have low emetogenicity and many institutions will give dexamethasone only prior to nab-paclitaxel to prevent nausea. In this trial, it was left to provider discretion but noted that about 80% of patients were given corticosteroids prior to their chemotherapy in both groups. Despite the corticosteroid premedication, patients still benefited from the addition of atezolizumab in progression-free survival (PFS) (7 months vs 5.5 months, p<0.0001) and overall survival (OS) (18.6 months vs 13.9 months, p=0.033).6
In the IMpower150 trial, treatment-naïve metastatic non-small-cell lung cancer patients were randomized to receive paclitaxel, bevacizumab and platinum plus or minus atezolizumab. Corticoste-roids are commonly used to prevent hypersensitivity reactions with paclitaxel, and this study left the management of corticosteroids to institution standard. Outcomes were favorable in the atezolizumab plus chemotherapy arm with improvement in PFS (8.3 months vs 6.8 months, p<0.001) and OS (19.2 months vs 14.7 months, p=0.02).7 In Keynote-021 and Keynote-189, non-small-cell lung cancer patients were randomized to receive pemetrexed plus platinum agents plus or minus pembrolizumab. Corticosteroids are commonly used to prevent rash from pemetrexed. As in the IMpower130 trial, the management of corticosteroids was left to institution standard. PFS and OS were favorable with the addition of pembrolizumab.
In Keynote-021G, PFS was improved at 24 months compared to 9.3 months in the chemotherapy alone arm (p=0.0049). OS was improved at 21.1 months in the chemotherapy alone arm and the median OS not yet reached in the pembrolizumab plus chemotherapy arm (p=0.0151).8 PFS was significantly better at 8.8 months versus 4.9 months (p<0.001) with addition of pembrolizumab in the Keynote-189 trial. OS also significantly improved at 11.3 months in the chemotherapy alone arm and median OS not yet reached (p<0.001) with the addition of pembrolizumab.9
In Keynote-407, non-small-cell lung cancer patients were randomized to receive a platinum agent, paclitaxel or nab-paclitaxel plus or minus pembrolizumab. It’s typical that nearly all patients on paclitaxel will get corticosteroids prior to their chemotherapy, at least for the first two doses. On the other hand, nab-paclitaxel, given its low emetogenicity and other options for anti-nausea, the corticosteroid may be omitted.
Unfortunately, the authors did not disclose the percentage of patients on corticosteroids with paclitaxel and nab-paclitaxel and this information was not able to be obtained. However, this study still showed that the treatment benefit of pembrolizumab was seen in PFS and OS. PFS was improved at 6.4 months versus 4.8 months (p<0.001) in the chemotherapy alone arm. OS was significantly better at 15.9 months versus 11.3 months (p<0.001) in the chemotherapy alone arm. Sixty percent of patients were on paclitaxel and it was found there was no difference in the treatment effect between the paclitaxel group and the nab-paclitaxel group.10
Data in Small-Cell Lung Cancer
In extensive-stage small-cell lung cancer, there are two trials supporting front-line indications. In the IMpower133 study, treatment-naïve patients were randomized to four cycles of carboplatin and etoposide with or without atezolizumab, followed by atezolizumab or placebo maintenance therapy. Premedications prior to chemotherapy were left to institution standard with a statement of caution to minimize corticosteroid use as much as possible given the theoretical effects of corticosteroids on ICI efficacy. The median OS was significantly improved in the atezolizumab group at 12.3 months vs 10.3 months (p=0.007). Furthermore, PFS was favorable in the atezolizumab group at 5.2 months versus 4.3 months (p=0.02).11
In the CASPIAN trial, durvalumab was evaluated in combination with the then standard of care chemotherapy regimen, a platinum (carboplatin or cisplatin) and etoposide. The addition of durvalumab provided a significant improvement in OS of 13 months versus 10.3 months (p=0.0047). In this study, premedications with corticosteroids was permitted prior to chemotherapy for prevention of nausea and vomiting.12
Data in Triple-Negative Breast Cancer
Keynote-522 evaluated pembrolizumab in combination with paclitaxel and carboplatin in previously untreated stage II or III triple-negative breast cancer patients. Patients received neoadjuvant therapy with four cycles of the pembrolizumab plus chemotherapy followed by four additional cycles of pembrolizumab or placebo alone. Both groups received four cycles of either doxorubicin and cyclophosphamide or epirubicin and cyclophosphamide every three weeks. After definitive surgery, patients received pembrolizumab or placebo alone for up to nine cycles.
Their first interim analysis was positive with a pathological complete response of 64.8% in the pembrolizumab plus chemotherapy group versus 51.2% in the chemotherapy alone group (p<0.001). The protocol left the premedications to institution standard, allowing corticosteroids prior to chemotherapy administration for prevention of nausea, vomiting, and hypersensitivity reactions.3
Guidelines
Prior to an update made this year, National Cancer Comprehensive Network (NCCN) guidelines contained a caveat when it comes to antiemetic use. In part, the guidelines said, “When ICI are administered concurrently with emetogenic chemotherapy, inconclusive data suggest concurrent corticosteroid administration may negatively impact cancer outcomes. Until more evidence is available, the panel recommends employment of a corticosteroid-sparing approach to antiemetic prophylaxis on a case-by-case and regimen basis.” This has been removed with the most recent 2020 update based on the multiple previous trials, which included concurrent corticosteroid use to prevent adverse effects from chemotherapy when combined with ICI13.
American Society of Clinical Oncology (ASCO) cites the two pembrolizumab trials (Keynote-021G and Keynote 189) completed in non-small-cell lung cancer patients as evidence that dexamethasone should not be removed from guideline-compliant antiemetic prophylaxis regimens used in chemotherapy plus ICI regimens.14Thus, the two leading oncology guidelines for antiemetic use supports the use of corticosteroids when appropriate prior to chemotherapy in combination with ICI.
Treatment Doses of Corticosteroids for Immune-related Adverse Effects
There has been the question of whether treatment of immune-related adverse events with corticosteroids impacts the efficacy of ICI. A retrospective review at Memorial Sloan Kettering Cancer Center looked at treatment of 103 patients that required systemic corticosteroids for their immune-related adverse events out of 254 patients who experienced immune-related adverse events. These doses of corticosteroids are usually as high as 1mg/kg of prednisone or equivalent but can vary widely between prescribing physicians.
Median time to treatment failure was 5.7 months and median OS was 16.5 months, which compared favorably with other ipilimumab studies. The time to treatment failure curve plateaued at 88%, leaving 12% who experienced long-term disease control despite the use of corticosteroids to treat immune-related adverse events. When patients were stratified by the presence or absence of immune-related adverse events of any grade, there was no difference in OS or time to treatment failure.
In addition, no difference in OS or time to treatment failure was observed when patients were stratified by administration of corticosteroids.15 Thus, high doses of corticosteroids used to treat immune-related adverse events do not appear to impact efficacy of immune-checkpoint inhibitors.
Baseline Corticosteroid Use
A physiologic dose of corticosteroids is approximately 7.5 mg of prednisone; therefore, doses less than or equal to 10 mg of prednisone have been deemed acceptable.16,17,18,19,20 Patients receiving more than 10 mg of prednisone or equivalent prior to and concurrently with immune-checkpoint inhibitors for longer durations than a few days have been excluded from trials thus far. There is some evidence that corticosteroid use prior to and within 30 days of initiation of immune-checkpoint inhibitors could impact efficacy.
A retrospective review of two cancer centers, Memorial Sloan Kettering and Gustrave Roussy reviewed 640 patients treated with single agent ICI. Ninety of these patients were on at least 10 mg of prednisone for various indications, including dyspnea, fatigue and brain metastasis. The overall response rates, PFS, and OS were significantly decreased in the corticosteroid group compared to the control group who had no steroids or less than 10 mg of prednisone on board. There was a similar detriment in efficacy with prednisone amounts greater than 20 mg versus 10-19 mg of prednisone.
They did find that the timing of discontinuation of the steroids had a varying impact on PFS and OS. When patients discontinued their corticosteroids at least one day prior to initiation of the ICI, they had intermediate PFS and OS. The best PFS and OS was seen in patients who had no corticosteroids within 30 days of therapy. Of note, authors adjusted for confounding factors, such as smoking history, performance status, and history of brain metastasis, and use of corticosteroids remained associated with decreased efficacy.21
Another retrospective review evaluated early use of corticosteroids in non-small-cell lung cancer patients treated with nivolumab monotherapy. The median daily dose of prednisone was 35 mg and went as high as 180 mg per day. Authors found that OS was significantly decreased at 11 months versus 4.3 months (p=0.017).22 These studies do have limitations and the design does not differentiate between correlation versus causation with baseline corticosteroids.
To answer this question, the Dana-Farber Cancer Institute com-pleted a retrospective review of 650 patients with non-small-cell lung cancer treated with single agent ICI. They categorized the indication for the corticosteroids as either palliative (cancer-related) or nonpalliative. Out of 650 patients, 93 were on at least 10 mg of prednisone to as high as 150 mg per day. Palliative indications included brain metastasis, cancer-related dyspnea, pain from bone metastasis, and cancer-related anorexia. Nonpalliative indications included, pneumo-nitis from prior treatment, chronic obstructive pulmonary disease, autoimmune disease and iodinated contrast prophylaxis.
There were significant differences in the baseline characteristics between the two groups. The performance status was poorer and the number of patients with brain metastasis prior to starting ICI was significantly higher. In those patients receiving corticosteroids for nonpalliative indications, the ICI was typically in the second-line or later. This could be significant as patients treated in the first line with ICI are expected to have better outcomes compared to patients being treated in subsequent lines of therapy.
After analysis, this review confirmed that baseline use of less than 10 mg of prednisone at the time of ICI initiation was associated with significantly lower overall response rates, PFS, and OS. However, when the indication for the corticosteroids was teased out, those patients on corticosteroids for nonpalliative indications had a similar PFS and OS compared to patients who were not on corticosteroids. Patients on corticosteroids for palliation still had significantly lower outcomes than patients not on corticosteroids. From this data, those patients on corticosteroids for cancer-related palliation had decreased efficacy likely due to an already poorer prognosis and not necessarily from the use of corticosteroids being concurrently administered with ICI.23
Although the mechanisms of corticosteroids are not fully elucidated, there is a theory regarding the potential mechanism of corticosteroids early administration in ICI treatment. In cancer, there is a state of CD8+ T-cell dysfunction that is associated with the expression of PD-1 inhibitory receptors. In a study in labs rats, it was found these PD-1 positive CD-8+ T-cells underwent self-renewal but mainly differentiated into terminally exhausted CD-8+ T-cells. When these mice were treated with PD-1 blockade, there was a proliferative burst almost exclusively of CD-8+ T-cells, resulting in restoration of their function. It is likely the benefit from ICI is largely derived from this initial burst in CD-8+ T-cells upon initiation of therapy. Therefore, the concern with corticosteroid use at baseline would blunt this T-cell burst and decrease the benefit.24 If true, the administration of cortico-steroids after this CD-8+ T-cell burst would not impact ICI efficacy.
Conclusion
Several trials have studied ICI in combination with chemotherapy and have allowed the use of corticosteroids to prevent adverse effects from chemotherapy. Outcomes have been favorable with the addition of ICI despite the use of corticosteroids. These trials are not conclusive that corticosteroids used to prevent adverse effects from chemotherapy do not have any impact on the efficacy of ICI, but they do show that the benefit of the addition of an ICI to chemotherapy is still appreciated despite the concurrent use of corticosteroids. It is appropriate for patients on these regimens to continue to receive corticosteroids to prevent adverse effects from chemotherapy.
REFERENCES
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 8.2020). https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed September 23rd, 2020.
- National Comprehensive Cancer Network. Small Cell Lung Cancer (Version 1.2021). https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed September 23rd, 2020.
- Schmid P, Cortes J, Pusztai L. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020;382:810-21.
- Greaves MW. Anti-inflammatory action of corticosteroids. Postgraduate Medical Journal (October 1976) 52, 631-633.
- Libert C and Dejager L. How steroids steer T cells. Cell Rep. 2014 May 22;7(4):938-9
- West H, McCleod M, Hussein M, et al. Atezolizumab in Combination with Carboplatin plus nab-paclitaxel Chemotherapy compared with Chemotherapy alone as First-line treatment for Metastatic non-squamous Non-small Cell Lung Cancer (IM power130): a multicentere, randomized, open-label, phase 3 trial. Lancet Oncol 2019; 20: 924–37.
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med2018;378:2288-301.
- Borghaei H, Langer CJ, Gadgeel S, et. al. 24-Month Overall Survival from Keynote-021 Cohort G: Pemetrexed and Carboplatin with or without Pembrolizumab as First-Line Therapy for Advanced Nonsquamous Non-Small-Cell Lung Cancer. J Thorac Oncol. 2019 Jan;14(1):124-129.
- Gandhi L, Rodriguez-abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med2018;378:2078-92.
- Paz-Ares L, Luft A, Vicente D, et. al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51.
- Horn L, Mansfield AS, Szczesna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med2018;379:2220-9.
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus Platinum-etoposide versus Platinum-etoposide in First-line Treatment of Extensive-stage Small-Cell Lung Cancer (CASPIAN): a randomized, controlled, open-label, phase 3 trial.
- National Comprehensive Cancer Network. Antiemesis (Version 2.2020). https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed September 23rd, 2020.
- Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: ASCO Guideline Update. J Clin Oncol 38:2782-2797.
- Horvat TZ, Adel NG, Dang TO et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients with Melanoma. J Clin Oncol. 2015 Oct 1;33(28):3193-8.
- Atezolizumab. Package Insert. Genentech, Inc; 2020.
- Durvalumab. Package Insert. AstraZeneca Pharmaceuticals LP; 2020.
- Ipilimumab. Package Insert. Bristol-Myers Squibb Company; 2018.
- Nivolumab. Package Insert. Bristol-Myers Squibb Company; 2020.
- Pembrolizumab. Package Insert. Merck & Co., Inc.; 2020.
- Arbour KC, Mezuita L, Long N, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients with Non-Small-Cell Lung Cancer. J Clin Oncol. 2018 Oct 1;36(28):2872-2878.
- Scott S and Pennel N. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J Thorac Oncol. 2018 Nov;13(11):1771-1775.
- Ricciuti B, Dahlberg S, Aden A. et al. Immune Checkpoint Inhibitor Outcomes for Patients with NSCLC Receiving Baseline Corticosteroids for Palliative versus Nonpalliative Indications. J Clin Oncol. 2019 Aug 1;37(22):1927-1934.
- Im SJ, Hashimoto M, Gerner MY, et al: Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537:417-421, 2016.
Exploring Teaching Opportunities During Residency Training
Alexandra Della Pia, PharmD MBA
Clinical Assistant Professor and Lymphoma Clinical Pharmacy Specialist
Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey
Hackensack University Medical Center
Hackensack, NJ
Matthew Daniels, PharmD
Bone Marrow Transplantation Clinical Pharmacy Specialist
Oklahoma University Medical Center
Oklahoma City, OK
With a variety of learning experiences during your residency year, teaching may not be a requirement. Yet, as a future clinical pharmacist, your primary role will be to serve as the medication expert for your service, meaning your day-to-day will most likely include teaching. You will teach your team about drug initiation or dose adjustments, educate patients about a new medication, and/or act as a teacher for the learners on your rotation. After residency, you may even be interested in pursuing clinical positions affiliated with pharmacy schools or residency programs, which will re-quire you to precept students and residents, respectively. Exploring teaching opportunities as a resident will help you begin to develop your teaching style and gain insight into potential career paths.
Selecting meaningful teaching opportunities can be challenging as a resident, especially when your time is limited, your interests are evolving, and you aren’t sure how to obtain these experiences. Below we share our thoughts on teaching opportunities available to residents.
Teaching Certificate Program
As a pharmacy resident, you may be given the opportunity to participate in a teaching certificate program with a local school of pharmacy. Depending on your level of interest in teaching (ranging from uncertain through passionate), participating in a teaching certificate program is a good place to start.
These programs vary in terms of topics and meeting, but generally offer lessons on how to write learning objectives, prepare lectures, create exam questions, and adapt your teaching style for different learners. Some of the benefits of participating in a teaching certificate program include the opportunity to create and present your own lecture to pharmacy students, as well as to gain skills and resources to be an effective preceptor or faculty member.
In addition, most teaching certificate programs encourage you to develop your personal teaching philosophy, which is something many residents have not yet written but may need when applying to faculty positions after residency. Lastly, you will have a lot of opportunities for networking with your colleagues since pharmacy residents from many programs in the surrounding area may participate in the teaching certificate program.
Precepting Pharmacy Students and/or PGY1 Pharmacy Residents
Depending on your residency year or site, you may be offered the opportunity to precept pharmacy students or postgraduate year one (PGY1) pharmacy residents. If precepting is not a requirement for your program, we suggest that you reach out to your residency pro-gram director (RPD) or faculty preceptors to let them know you are interested so they can help to identify times of the year when you may be able to precept learners. There are many ways to get involved with precepting based on your desire to teach and the amount of time you have to commit amongst your residency projects and commitments.
If you do not feel you can precept a rotation due to time intensity, then precepting a student or resident on an in-service, journal club, or case presentation may be right for you. Precepting these types of learning experiences is often less cumbersome as it mainly involves reviewing drafts, providing feedback, and being present to support your learner on the day of his or her presentation.
If you are more passionate about teaching or trying to further develop your teaching style, then precepting a student or resident on a rotation may be more fruitful for you. Serving as a rotation preceptor will challenge you to meet the learner at his or her level of understanding and help you find different ways to describe processes, mechanisms of action, and disease state etiologies. Not only does this help the student or resident learn about managing a new or complex disease state, but also helps you cement the information you’re learning much faster. Many residents decline precepting opportunities due to a lack of confidence early on in the residency year (trust us, we’ve been there), but we encourage you to take these opportunities when they are presented. You most likely know more than you give yourself credit for!
In-service Presentations
In-service presentations are a great way to incorporate a teaching experience into your residency year, especially if other opportunities (such as a teaching certificate program or precepting) are not available. In-service presentations are shorter presentations based on the needs and interests of your audience, and can be given to multidisciplinary teams, nurses, and staff pharmacists. As a future clinical pharmacist, your primary role will be to serve as the medication expert; your day-to-day will likely include teaching your team and patients.
This teaching opportunity is a beneficial way to practice disseminating information according to your target audience. For example, when giving an in-service presentation to nurses, you may focus on monitoring parameters, side effects, and drug administration while only briefly mentioning dosing and drug interactions. On the other hand, when providing an in-service presentation to staff pharma-cists, it may be more beneficial to elaborate on dose adjustments, indications, drug interactions, and other nuances to help with order verification. In-service presentations can help further develop your teaching skills by challenging you to think of a topic from a different point of view and anticipate what information your audience may want to know.
Miscellaneous Teaching Experiences
There are a few other teaching opportunities that may be part of your residency program or about which you can ask your RPD and preceptor. One way to have a more formal teaching experience is to present an Accreditation Council for Pharmacy Education (ACPE) seminar. This accredited seminar can be presented to the pharma-cy department at your hospital or through a third party (such as a pharmacy school or conference) where attendees received continuing education (CE) credit.
Some of the benefits of presenting an ACPE-accredited seminar include presenting to a larger audience, creating assessment questions to gauge audience comprehension, and becoming the expert on a topic. You will review current literature and comment on its application to clinical practice. Another way to get teaching experience is to reach out to faculty preceptors or mentors for the chance to teach a lecture during a pharmacy school course or lead a student seminar for students on rotation. These are both great ways to gain experience teaching students, further develop your teaching style, and learn more about a career in academia.
In summary, there are many ways to explore teaching opportunities as a pharmacy resident. Whether you are uncertain about your interest in teaching or hope to be a preceptor someday, we suggest including at least one of these experiences in your residency year. The skills you develop will undoubtedly help you in your future career.
Feature: 2020 View on Updates in the Treatment of Lung Cancer
Chung-Shien Lee, PharmD BCOP BCPS
Assistant Professor St. John’s University College of Pharmacy
Queens, NY
Lung cancer remains the deadliest form of cancer in the United States, accounting for approximately 135,720 deaths with an estimated 228,850 new cases in 2020.1 Lung cancer can be classified as two major histological groups, small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Treatment of NSCLC has become dependent on molecular profiling since many cases harbor a drivergenetic alterations, most notably mutations in the epidermal growth factor receptor (EGFR) or rearrangements of the anaplastic lymphoma kinase (ALK) gene and ROS1 genes.2,3 The year 2020 was a year of tremendous growth for lung cancer treatment with several new drug approvals and lung cancer trials maturing.
Treatments Targeting ALK
ALK rearrangements are present in 3-5% of NSCLC and are effectively treated with ALK tyrosine kinase inhibitors (TKI).4,5,6
Brigatinib
Brigatinib is a second-generation ALK TKI that was originally approved for the treatment of patients with metastatic ALK-positive NSCLC who had progressed on or were intolerant to crizotinib.7,8 Subsequently, brigatinib received approval as first-line therapy in ALK-positive metastatic NSCLC in May 2020 as a result of the AL-TA-1L trial.9,10 This was an open-label trial, evaluating 275 patients with locally advanced or metastatic ALK-positive NSCLC who were naïve to ALK-targeting therapy. Patients were randomized to receive either brigatinib (n=137) or crizotinib (n=138). The primary endpoint was progression-free survival (PFS). The estimated median PFS was 24.0 months (95% CI, 18.5 to not reached) in the brigatinib group compared to 11.0 months (95% CI, 9.2 to 12.9) in the crizotinib group [HR=0.49, (95% CI, 0.35 to 0.68); p<0.0001]. This benefit was consistent across subgroups, including those with baseline brain metastases.9 The NCCN clinical practice guidelines currently recommend brigatinib as a first-line option in patients with an ALK rearrangement.11
Ensartinib
Ensartinib is a second-generation ALK TKI that was recently shown to be superior to crizotinib. Interim results of eXalt3 study, which was a randomized, open-label, phase III study was presented at the IASLC World Conference on Lung Cancer Virtual Presidential Symposium.12 Ensartinib demonstrated a benefit in the primary endpoint, median PFS (25.8 months vs 12.7 months) with a 49% reduction in the risk of disease progression or death (HR=0.51, 95% CI, 0.35 to 0.72; p=0.0001) in patients with locally advanced or metastatic ALK-positive NSCLC who were naïve to ALK-targeting therapy.
Other efficacy outcomes, such as duration of response and overall survival were not yet mature, but favored ensartinib. In addition, ensartinib demonstrated CNS activity in a small subgroup. Ensartinib had similar rates of serious treatment-related adverse events (TRAEs) (8% vs 6%), dose reductions (24% vs 20%), and drug discontinuations (9% vs 7%) compared to crizotinib.12 The results of the eXalt3 study demonstrate ensartinib as a potential new first-line treatment option for patients with ALK-positive NSCLC.
Treatments Targeting EGFR
EGFR mutations have been found in up to 50% of Asian patients with NSCLC.13 Deletion in exon 19 (45%) and L858R point mutation in exon 21 (40%) are the two most common types of mutations found.14,15 EGFR TKIs have shown to be an effective treatment option for these patients.
Osimertinib
Osimertinib is a third-generation EGFR TKI that was approved as first-line therapy in patients with metastatic EGFR mutated NSCLC in 2018. This approval was based on results of the FLAURA trial, which was a double-blind, phase III trial that demonstrated an improvement in PFS with osimertinib compared to first-generation EGFR TKIs (gefitinib or erlotinib) in 556 patients with advanced EGFR mutated NSCLC. The median PFS was found to be18.9 months and 10.2 months, respectively; HR=0.46; 95% CI, 0.37 to 0.57; p<0.001).16 Recently, long-term follow up of this study also demonstrated an improvement in OS with a median OS of 38.6 months in the osimertinib group compared to 31.8 months in the first-generation EGFR TKI group (HR=0.80, 95.05% CI, 0.64 to 1.00; p=0.046).17
After showing benefit in the advanced EGFR mutated NSCLC setting, osimertinib was investigated in the adjuvant setting. Recently, results of the ADAURA study were disseminated. This was a randomized, double-blinded, placebo-controlled phase III trial comparing osimertinib to placebo in patients with EGFR mutated NSCLC in the adjuvant setting. There was an 83% improvement in disease free survival (DFS) with the osimertinib group (HR=0.17, 95% CI, 0.12 to 0.23; p<0.0001) in stage II to IIIA patients. When patients with stage IB NSCLC were added to the analysis, osimertinib improved DFS by 79% (HR=0.21, 95% CI, 0.16 to 0.28; p<0.0001).18 These results demonstrate osimertinib effectiveness in the adjuvant setting with patients with EGFR mutated NSCLC.
Ramucirumab + Erlotinib
The combination of ramucirumab and erlotinib is the first approval of a vascular endothelial growth factor receptor (VEGFR) inhibitor with an EGFR TKI for first-line treatment of metastatic EGFR mutated NSCLC. The RELAY trial was a randomized, double-blind, placebo-controlled phase III trial investigating the addition of ramucirumab to erlotinib in treatment naïve, EGFR mutated, advanced NSCLC.
The primary endpoint of PFS was significantly longer in the combination group [19.4 months (95% CI, 15.4 to 21.6)] compared to the erlotinib alone group [12.4 months (95% CI, 11.0 to 13.5)] [HR=0.59 (95% CI, 0.46 to 0.76; p<0·0001)]. Severe TRAEs were higher in the combination group compared to the erlotinib alone group (72% vs 54%). The most common severe TRAEs in the ramucirumab plus erlotinib group were hypertension (24%) and derma-titis acneiform (15%).19 Currently the combination of ramucirumab and erlotinib is an option for patients with advanced EGFR mutated NSCLC, but osimertinib is the preferred option according to the NCCN guidelines.11
Treatments Targeting RET
RET rearrangements are less common than ALK rearrangements and EGFR mutations and have been reported to be present in 1-2% of NSCLC cases.20SelpercatinibSelpercatinib is a novel, highly selective RET TKI that was granted accelerated approval by the FDA for adult patients with metastatic RET fusion-positive NSCLC and select patients with RET-mutant or RET fusion-positive thyroid cancer. This approval was based on the results of the LIBRETTO-001 study, which was a phase 1/2 trial that included patients 12 years of age or older with a RET positive advanced or metastatic solid tumor. Drilon and colleagues reported the results for 144 patients with NSCLC (39 previously untreated and 105 patients who had received at least platinum-based chemo-therapy). Among the patients who had previously received treatment, many were heavily pretreated with a median of three previous treatments (range, 1 to 15) and over half previously received immunotherapy. Previously treated patients had an objective response rate of 64% (2% complete response) and a median duration of response of 17.5 months [95% CI, 12.0 to not estimable (NE)]. In the treatment naïve group, the objective response rate (ORR) was 85%. The median PFS was 16.5 months (95% CI, 13.7 to NE) in all patients. Selpercatinib was generally well tolerated with the most common TRAEs being dry mouth (36%), diarrhea (25%), increased liver enzymes (20-22%) and hypertension (17%). The most com-mon severe TRAEs were hypertension (14%) and increased liver enzymes (6-9%). Four patients discontinued selpercatinib due to TRAEs.21 Selpercatinib is currently a preferred option for advanced NSCLC with a RET rearrangement.11PralsetinibSimilar to selpercatinib, pralsetinib is a highly selective RET TKI that was granted accelerated approval by the FDA for adult patients with metastatic RET fusion-positive NSCLC. The phase I/II ARROW trial was a multicenter, open-label, multi-cohort clinical trial evaluating the use of pralsetinib in patients with advanced RET fusion-positive solid tumors. In a recent report, 87 NSCLC patients previously treated with platinum-based chemotherapy had an ORR of 57% (95% CI, 46% to 68%). Median duration of response was not estimable (95% CI, 15.2 months to not estimable). The ORR was 70% (95% CI, 50% to 86%) with a median duration of response of 9.0 months (95% CI, 6.3 months to not estimable) in 27 treatment-naive patients who were ineligible for platinum-based chemotherapy. The most common TRAEs included increased aspartate aminotransferase (31%), anemia (22%), increased alanine amino-transferase (21%), constipation (21%) and hypertension (20%). Four percent of patients discontinued pralsetinib due to TRAEs.22,23Pralsetinib is currently a preferred option for advanced NSCLC with a RET rearrangement.11
Treatments Targeting Mesenchymal-epithelial transition (MET)
Recently, MET has presented itself as another actionable tar-get mutation. MET exon 14 skipping mutations occur in 3-4% of NSCLC patients and MET amplification occurs in 1-6% of NSCLC patients.24,25
Capmatinib
Capmatinib is a potent, selective MET TKI that was granted accelerated approval by the FDA for adult patients with metastatic NSCLC whose tumors have a mutation that leads to MET exon 14 skipping. This approval was based on the GEOMETRY mono-1 study, which was an open-label, multiple-cohort, phase 2 study that investigated the use of capmatinib in patients with advanced NSCLC with a MET exon 14 skipping mutation or MET amplification. The primary end point was ORR, which was 68% (95% CI, 48 to 84) in 28 patients who were treatment naïve and 41% (95% CI, 29 to 53) in 69 patients who were previously treated. Median duration of response was 12.6 months (95% CI, 5.6 not estimable) and 9.7 months (95% CI, 5.6 to 13.0), respectively. Median PFS was 12.4 months (95% CI, 8.2 to not estimable) and 5.4 months (95% CI, 4.2 to 7.0), respectively. There was a limited response in previously treated patients with MET amplification who had a gene copy number of <10 orr="7-12%)." the="" most="" commonly="" reported="" adverse="" events="" were="" peripheral="" edema="" 51="" nausea="" 45="" increase="" serum="" creatinine="" 24="" and="" dyspnea="" 23="" which="" mostly="" of="" grade="" 1="" or="" 2="" sup="">26 Capmatinib is currently the preferred option for advanced NSCLC with a MET mutation.11
Tepotinib
Tepotinib is a selective MET inhibitor that was recently shown to be efficacious in advanced NSCLC patients with MET exon 14 skipping mutations. The VISION study was a multicohort, open-label, phase II study evaluating tepotinib in advanced NSCLC patients with MET alterations. One hundred and fifty-two patients were treated with tepotinib (safety population) and out of these, 99 had a confirmed biopsy (liquid or tumor) and at least 9 months of follow-up (efficacy population). The primary end point was confirmed ORR, which was 46% (95% CI, 36 to 57) with no complete responses. Responses were similar in both biopsy groups, 48% (95% CI, 36 to 61) in the liquid biopsy group and 50% (95% CI, 37 to 63) in the tissue biopsy group. Median PFS was 8.5 months (95% CI, 6.7 to 11.0). The most commonly reported adverse events were peripheral edema (63%), nausea (26%) and diarrhea (22%) with 7% of patients experiencing FEATURE (continued)24FEATURE (continued)severe peripheral edema. The VISION study demonstrated tepotinib to be a well-tolerated efficacious agent for advanced NSCLC patients with MET exon 14 skipping mutation.27
Lurbinectedin for SCLC
Lurbinectedin is an alkylating agent and a selective inhibitor of oncogenic transcription, which binds preferentially to guanines located in the GC-rich regulatory areas of DNA gene promoters leading to tumor cell apoptosis. It was granted accelerated approval for adult patients with metastatic SCLC with disease progression on or after platinum-based chemotherapy.
In a single-arm, open-label, phase II basket trial, lurbinectedin demonstrated an overall response of 35.2% (95% CI, 26.2 to 45.2) in 105 patients who were pre-treated with only one previous chemo-therapy containing treatment line (immunotherapy was allowed). The most common adverse events observed were anemia (87%), elevated creatinine (83%), elevated alanine aminotransferase (67%), leucopenia (50%), elevated γ-glutamyl transferase (50%), fatigue (50%), elevated aspartate aminotransferase (43%), and thrombocytopenia (37%). Severe adverse events were mostly hematological.28 Lurbinectedin is currently the preferred regimen for SCLC patients who have relapsed within 6 months of first line treatment.29
Durvalumab for SCLC
Durvalumab is a PD-L1 inhibitor, which was approved as first-line treatment of patients with extensive-stage SCLC in combination with etoposide and a platinum agent. The CASPIAN trial was a randomized, open-label, phase III trial that investigated durvalumab in treatment naïve extensive-stage SCLC. Durvalumab plus platinum etoposide demonstrated a significant improvement in OS compared to platinum etoposide alone [HR = 0.73 (95% CI, 0.59 to 0.91; p=0·0047)]; median OS of 13.0 months (95% CI, 11.5 to 14.8) vs 10.3 months (95% CI, 9.3 to 11.2). The rate of severe adverse events was similar in the two groups.30 Durvalumab plus platinum etoposide is currently a preferred first line regimen for extensive-stage SCLC patients.29
Immunotherapy for NSCLC
Immunotherapy has become the mainstay of treatment for patients with NSCLC in the absence of a driver mutation.11 Several approvals with various immunotherapy agents have occurred in this patient population in 2020:
- Nivolumab plus ipilimumab was approved as first-line treatment for patients with metastatic NSCLC whose tumors express ≥1% PD-L1 with no EGFR or ALK genomic tumor aberrations.31
- Atezolizumab was approved as first-line treatment of adult patients with metastatic NSCLC whose tumors have high PD-L1 expression (PD-L1 stained ≥ 50% of tumor cells [≥ 50%] or PD-L1 stained tumor-infiltrating immune cells [IC] covering ≥ 10% of the tumor area [IC ≥ 10%]), with no EGFR or ALK genomic tumor aberrations.32
- Nivolumab plus ipilimumab was approved with 2 cycles of platinum doublet chemotherapy as first-line treatment for patients with metastatic or recurrent NSCLC with no EGFR or ALK genomic tumor aberrations.33
Data from the phase III ORIENT-11 trial was recently presented at the IASLC Virtual Presidential Symposium 2020. This trial demonstrated sintilimab, an anti-PD-1 inhibitor, when added to pemetrexed and platinum-based therapy significantly improved PFS compared to chemotherapy alone in patients with locally advanced or metastatic nonsquamous NSCLC. Median PFS was 8.9 months (95% CI, 7.1 to 11.3) compared to 5.0 months (95% CI, 4.8 to 6.2), respectively (HR=0.482, 95% CI, 0.362 to 0.643]; p<0.00001). This benefit was seen across all subgroups and increased with higher PD-L1 tumor expression. Sintilimab was well tolerated, with similar incidence of overall adverse events (AEs; 99.6% vs 100%), severe adverse events (61.7% vs 58.8%), and adverse events leading to treatment discontinuation (6.0% vs 8.4%).34 Sintilimab provides a treatment option for patients with nonsquamous NSCLC with no EGFR mutations or ALK rearrangements, where treatment options are limited.
Conclusion
A plethora of much-needed, new treatment options and approvals for lung cancer have occurred in 2020. These include various oral TKIs for driver mutations, as well as combination immunotherapy treatments. As further data matures, the treatment paradigm for SCLC and NSCLC will continue to shift and improve patient out-comes.
REFERENCES
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020 Jan;70(1):7-30. doi: 10.3322/caac.21590. Epub 2020 Jan 8.
- Pakkala S, Ramalingam SS. Epidermal Growth Factor Receptor Mutated Advanced Non-Small Cell Lung Cancer: A Changing Treatment Paradigm. Hematol Oncol Clin North Am. 2017 Feb;31(1):83-99. doi: 10.1016/j.hoc.2016.08.003.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017.
- Gainor JF, Varghese AM, Ou SH, et al: ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: An analysis of 1,683 patients with nonsmall cell lung cancer. Clin Cancer Res 19:4273-4281, 2013
- Wong DW, Leung EL, So KK, et al: The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 115:1723-1733, 2009
- Koivunen JP, Mermel C, Zejnullahu K, et al: EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 14:4275-4283, 2008
- Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(12):1683-1696. doi:10.1016/S1470-2045(16)30392-8
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol. 2017;35(22):2490-2498. doi:10.1200/JCO.2016.71.5904
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor-Naive ALK-Positive Non-Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial [published online ahead of print, 2020 Aug 11]. J Clin Oncol. 2020;JCO2000505. doi:
- 1200/JCO.20.0050510. Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379(21):2027-2039. doi:10.1056/NEJMoa1810171
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer. Version 8.2020 (September 15, 2020). Available at https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Selvaggi G, Wakelee HA, Mok T, et al. Phase III randomized study of ensartinib vs crizotinib in anaplastic lymphoma kinase (ALK) positive NSCLC patients: EXALT3. 2020 World Conference on Lung Cancer. Presidential Symposium. Abstract 2.
- Hirsch FR, Bunn PA Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009 May;10(5):432-3. doi: 10.1016/S1470-2045(09)70110-X.
- Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006 Dec 15;12(24):7232-41.
- O’Kane GM, Bradbury PA, Feld R, et al. Uncommon EGFR mutations in advanced non-small cell lung cancer. Lung Cancer. 2017 Jul;109:137-144. doi: 10.1016/j.lungcan.2017.04.0
- Epub 2017 Apr 27.16. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018 Jan 11;378(2):113-125. doi: 10.1056/NEJMoa1713137. Epub 2017 Nov 18.
- Ramalingam SS, Vansteenkiste J, Planchard D, FLAURA Investigators. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med. 2020 Jan 2;382(1):41-50. doi: 10.1056/NEJMoa1913662. Epub 2019 Nov 21.
- Herbst RS, Tsuboi M, John T, et al. Osimertinib as adjuvant therapy in patients with stage IB-IIIA EGFR mutation positive NSCLC after complete tumor resection: ADAURA. J Clin Oncol 38: 2020 (suppl; abstr LBA5). doi: 10.1200/JCO.2020.38.18_suppl.LBA5.
- Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(12):1655-1669. doi:10.1016/S1470-2045(19)30634-5
- Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18(3):378-381. Published 2012 Feb 12. doi:10.1038/nm.2658
- Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383(9):813-824. doi:10.1056/NEJMoa2005653
- Gainor JF, Curigliano G, Kim D-W, et al. Registrational dataset from the phase I/II ARROW trial of pralsetinib (BLU-667) in patients (pts) with advanced RET fusion+ non-small cell lung cancer (NSCLC). J Clin Oncol 38: 2020 (suppl 9515-9515). doi: 10.1200/JCO.2020.38.15_suppl.9515.
- U.S. Food and Drug Administration. FDA approves pralsetinib for lung cancer with RET gene fusions. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pralsetinib-lung-cancer-ret-gene-fusions?utm_medium=email&utm_source=govdelivery. Accessed September 11, 2020.
- Awad MM, Oxnard GR, Jackman DM, et al. MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J Clin Oncol. 2016;34(7):721-730. doi:10.1200/JCO.2015.63.4600
- Onozato R, Kosaka T, Kuwano H, Sekido Y, Yatabe Y, Mitsudomi T. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J Thorac Oncol. 2009;4(1):5-11. doi:10.1097/JTO.0b013e3181913e0e
- Wolf J, Seto T, Han JY, et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383(10):944-957. doi:10.1056/NEJMoa2002787
- Paik PK, Felip E, Veillon R, et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med. 2020;383(10):931-943. doi:10.1056/NEJMoa2004407
- Trigo J, Subbiah V, Besse B, et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020;21(5):645-654. doi:10.1016/S1470-2045(20)30068-1
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer. Version 1.2021 (August 11, 2020). Available at https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929-1939. doi:10.1016/S0140-6736(19)32222-6
- U.S. Food and Drug Administration. FDA approves nivolumab plus ipilimumab for first-line mNSCLC (PD-L1 tumor expression ≥1%). Available at https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-nivolumab-plus-ipilimumab-first-line-mnsclc-pd-l1-tumor-expression-1
- U.S. Food and Drug Administration. FDA approves atezolizumab for first-line treatment of metastatic NSCLC with high PD-L1 expression. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-first-line-treatment-metastatic-nsclc-high-pd-l1-expression
- U.S. Food and Drug Administration. FDA approves nivolumab plus ipilimumab and chemotherapy for first-line treatment of metastatic NSCLC Available at https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-nivolumab-plus-ipilimumab-and-chemotherapy-first-line-treatment-metastatic-nsclc
- Zhang L, Yang Y, Wang Z, et al. ORIENT-11: Sintilimab + pemetrexed + platinum as first-line therapy for locally advanced or metastatic non-squamous NSCLC. 2020 World Conference on Lung Cancer. Presidential Symposium. Abstract 1.
A Novel Book Club Regimen
Marin Abousaud, PharmD
Oncology Clinical Pharmacy Specialist, Head and Neck Cancer
Emory Healthcare, Winship Cancer Institute
Atlanta, GA
I have been very fortunate to have encountered several inspiring oncology patients throughout my training and career as an oncology clinical pharmacy specialist. There is one patient in particular who has made a lasting impact on the way I practice today.
During my fourth year of pharmacy school, I had an outpatient oncology rotation in an outpatient infusion center in a community cancer clinic. On my first week there, I met an older patient who was newly diagnosed with an oral cavity tumor. While my preceptor was educating her on the chemotherapy regimen and supportive care measures, I noticed she was overwhelmed with emotion. Afterwards, I engaged her in conversation and tried to provide comfort about her new diagnosis and treatment anxieties.
She shared with me that her husband had recently passed away, and she had been very lonely and depressed. They used to read books together and have long discussions about them. Since she was coming in for treatment each week – and sometimes, several times per week – I convinced her that we should start a book club and discuss the chapters when she came in for her treatments. As I built rapport with her, I was able to make recommendations that were tailored to her care and convinced her to seek treatment for her depression. She trusted my supportive care recommendations for her nausea and tried her best to increase her water intake since I reminded her about it each time I saw her.
Week after week, it was evident her morale was improving. Whenever she walked into the infusion area and saw me, she had a huge smile on her face and started waving her book, expressing her excitement to begin our discussion. By the end of my five weeks, we were able to get through two books; one she had suggested and another I suggested. On our last day together, I expressed how grateful I was to meet her and how deeply she had impacted my journey. I will never forget this next moment – she began tearing up and stated that I was the only reason she looked forward to her treatment appointments and that my positive, supportive attitude was what uplifted her spirits and helped her through one of the darkest moments in her life.
It was then that I truly understood the impact pharmacists, especially within the oncology field, have on patients. We see patients during some of their most challenging moments, when the stress, anxiety, fear, uncertainty, and toxicities they endure can be very overwhelming for them. Taking the time to build relationships with patients, understand their wants and needs, and provide support for them during these difficult times allows us to provide the best possible care. To this day, I still have a copy of the books we read together, and they serve as nice reminders to remain the positive, supportive person and pharmacist that my patients need.
Discovering Inspiration and Independence as a Clinical Pharmacist through Patient Interactions
Raymond DeMatteo, PharmD
Clinical Pharmacy Specialist
Memorial Sloan Kettering Cancer Center
New York, NY
As trainees, we learn to optimize pharmacotherapy to ensure appropriate outcomes for our patients. What is not always expected is the emotional bond we form with them, and the technical and operational aspects of our jobs that allow us to act as advocates for them. I didn’t fully appreciate these concepts until I entered my postgraduate year two (PGY2) in oncology, where I encountered a memorable patient on my leukemia rotation.
My patient was a young man diagnosed with chronic myeloid leukemia who had been placed on dasatinib. His BCR-ABL transcripts fluctuated due to non-compliance. Two weeks before he arrived to our service, he was admitted to an outside hospital and was found to be in lymphoid blast crisis. After a complicated hospital stay, he was discharged on ponatinib and presented to our team for systemic chemotherapy.
When I met with the patient and his mother to review the chemotherapy regimen, I remember the worry in their eyes, but also how kind and friendly they were. After spending 30-40 minutes discussing the treatment, they seemed more at ease and expressed gratitude for the information. It was at this time that I inquired how much ponatinib they had left, and they informed me that they were almost out. I had the physician send a new prescription to his pharmacy, but issues arose when insurance was not able to process the prescription. I spent hours on the phone with the insurance company, insisting that the request be expedited. After calling daily for 5 days, the medication was approved, and we avoided any missed doses.
This victory seemed short lived as the patient developed complications to chemotherapy, and I was asked to research the use of an antidote to help further mitigate toxicity. Hours of work were spent assessing the proper use, dose, monitoring parameters and procurement of the drug. Fortunately, the patient improved with supportive care alone, and did not need the antidote.
I learned several lessons from this patient experience, the first being that our responsibilities as clinical specialists extend to the operational aspects of care to ensure our patients can get their medications in a timely manner. If I had not spent hours on the phone with insurance, he would have experienced a treatment delay. Furthermore, it is a reminder of how valuable we are to the healthcare team when chemotherapy complications occur. I had never before been put in a situation where I needed to formulate a plan of action to prevent further toxicity, and it served as a fundamental lesson that these instances occur frequently in our profession and our expertise is needed to ensure medication safety.
This patient will always stay with me, as it’s a reminder of the value pharmacists bring to the healthcare team. It is our role to ensure patients receive the best pharmaceutical care possible. It is interactions like these that drive us as practitioners to optimize patient care and serve as patient advocates.
A Humane Hello
Emmeline C. Academia, PharmD
Clinical Pharmacy Specialist, Hematology/Oncology
Beth Israel Deaconess Medical Center
Boston, MA
It was another busy afternoon in the gastrointestinal oncology clinic, where pharmacists provide drug information, toxicity checks, and patient education for oral and intravenous chemotherapy. I was three months into my postgraduate year one (PGY1) training and on my ambulatory medical oncology rotation when a physician asked me to provide educa-tion for a pancreatic cancer patient.
The initial plan was for neo-adjuvant chemotherapy and surgery, but the tumor did not shrink as desired. Both the surgeon and medical oncologist presented treatment options to the patient and they decided to try to get to surgery with a different regimen. Leaning on a pragmatic optimism, I took my education material and talking points and walked into the examination room to meet the patient and his wife.
I knocked, entered, and introduced myself, smiling and ready to lead the conversation with confidence. After a “Good afternoon,” I asked a quick and nonchalant but happy, “How are you?” His wife smiled in a subdued optimism, nervously anticipating the conversation. The patient looked down in forlorn disappointment before slowly lifting his head to muster a weak smile with a heavy-chested sigh. Both of their faces, for a second, expressed a slightly offended, “How do you think we are?”
Everyone has little pet peeves, and one of mine is using “How are you?” as a synonym to “Hello.” It’s my opinion that “How are you?” is not a salutation; if you are walking down a hallway without intent to stop and hear the response – it’s not meant to be a greeting. Now, enter my inner dialogue: “Really? ‘How are you?’ The phrase that irks you on a normal day? And to a cancer patient no less? Who just received news that treatment didn’t work, and who has had all of an hour to process this new information?”
After completing my inner conversation, I paused and re-grouped. I apologized for the promptness of my salutation. We chatted for a few minutes about their frustration and sadness around the lack of tumor response, alluding to a fear that the next round wouldn’t work and dancing around the idea of losing hope. He stopped to breathe, in tedious disbelief and ponderous cliché as he asked if this was normal: “Why me?”
“Some things we expect, but other things, we just don’t understand well enough yet.”
“Okay. Well, how will this be different...?” We talked about the new chemotherapy plan, side effects, and potential interactions with the long list of herbal supplements he had brought with him. Their questions were ad-dressed; and perhaps, after a little ambiguity was made clear, a little hope was reinstilled.
That day reminds me that the provision of healthcare, especially in oncology, is always a two-way street. We can get so wrapped up in providing information in our busy days that sometimes, we forget that someone has to receive it. This patient reminded me of the humanity in our work, that no matter how busy we are, our duty is to provide accurate information thoughtfully. This mindful engagement of our patients is part of the quality that an ambulatory oncology pharmacist provides. That day, my practice was re-framed with a simple but meaningful rule: make sure your patients are, and feel, heard.
Conventional Versus Liposomal Irinotecan for Advanced Pancreatic Cancer
Justin C. Tossey, PharmD BCOP
Hematology/Oncology Clinical Specialist Pharmacist
The Ohio State University Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute
Columbus, OH
Pancreatic cancer remains one of the leading causes of cancer-related death in the U.S., despite being only the eleventh-most common new cancer diagnosis. The vast majority of pancreatic cancers are diagnosed at an advanced stage—either locally advanced and not surgically resectable or metastatic—so treatment relies primarily on systemic chemotherapy.
Over the last decade, research has led to increased survival using chemotherapy combinations such as FOLFIRINOX (leucovorin, 5-fluorouracil, irinotecan, and oxaliplatin) or gemcitabine and nab-paclitaxel as first-line treatment options. More recently, nanoliposomal irinotecan (nal-iri), a novel formulation, gained FDA approval in 2015 for the treatment of advanced pancreatic cancer in combination with leucovorin and 5-fluorouracil (5FU) after progression on gemcitabine-based therapy based on the results of the multinational phase III NAPOLI-1 trial.1
Prior to nal-iri/5FU, there was no systemic therapy specifically approved beyond first-line treatment for advanced pancreatic cancer. Treatment recommendations were extrapolated from the activity of first-line regimens and included a fluoropyrimidine combined with either oxaliplatin or conventional irinotecan (i.e. FOLFOX or FOLFIRI, respectively) after first-line gemcitabine-based treatment. Studies of FOLFIRI in this setting have often been retrospective in nature, though a phase II study comparing FOLFIRI and FOLFOX for second-line treatment found similar overall survival of about 4 months.2 To date, no randomized clinical trials have compared nal-iri/5FU to FOLFIRI, so it is unknown whether one regimen is clinically superior to the other.
Preclinical data demonstrate a 5-fold higher intratumoral concentration of the active irinotecan metabolite SN-38 after nal-iri administration compared to conventional irinotecan.3 This raises the intriguing possibility that nal-iri may be preferentially taken up by tumor cells, thereby increasing tumor cell killing while sparing normal cells from the toxic effects of SN-38. However, the available data suggest FOLFIRI may achieve survival outcomes similar to the more expensive nal-iri/5FU regimen. The increased cost of nal-iri/5FU could be justified if shown to have superior efficacy or significantly less toxicity than conventional irinotecan. Therefore, we sought to address this gap in clinical knowledge.
We retrospectively reviewed medical records of adult patients with locally advanced or metastatic pancreatic cancer who received either nal-iri/5FU or FOLFIRI after a gemcitabine-based therapy from October 2015 to August 2018.4 The primary outcome of our study was progression-free survival (PFS), with secondary endpoints that included time to treatment failure (TTF), overall survival (OS), frequency of dose reductions or treatment delays, and frequency of adverse effects. We also incorporated a cost analysis using estimates based on data available from the Centers for Medicare and Medicaid Services (CMS).
To account for potential differences between groups, we utilized inverse probability of treatment weighting (IPTW) based on baseline characteristics, and applied IPTW adjustment to all outcomes. We did not conduct any statistical hypothesis tests since the study hypothesis was non-inferiority of FOLFIRI and the limited sample size did not provide adequate power for formal tests of non-inferiority.
A total of 82 patients were screened for inclusion, and patients were excluded for not having received prior gemcitabine (n = 5) or not having received a study treatment (n = 2). Of the remaining 75 patients, 35 received nal-iri/5FU and 40 received FOLFIRI. After IPTW adjustment, treatment groups were balanced with regard to all baseline characteristics. Nearly all patients (88%) had metastatic disease, with 71% of those patients having hepatic involvement. More than half of patients in each group received at least 2 prior systemic therapies. Approximately one-third (n=23) of patients had prior exposure to irinotecan, mostly in the neoadjuvant setting (7 of 10 nal-iri/5FU patients and 11 of 13 FOLFIRI patients).
The primary outcome of PFS was similar between treatment groups, with a median of 4.1 months for nal-iri/5FU and 3.1 months for FOLFIRI. OS was also similar (7.1 vs 6.7 months), while TTF was nearly 2 months longer for nal-iri/5FU (4.1 months) com-pared to FOLFIRI (2.2 months). Treatment delays were common in the nal-iri/5FU group (66% vs 36%), whereas dose reductions occurred more frequently in the FOLFIRI group (48% vs 39%). Granulocyte colony-stimulating factor (G-CSF) use was similar between both groups (16% vs 15%), but atropine for the management of acute diarrhea (we do not regularly utilize atropine for primary prophylaxis) was used in nearly twice as many patients in the FOLFIRI group (70% vs 36%). There were no distinct differences in the frequency of grade 3 or 4 adverse effects. The cost analysis based on CMS data estimated a total treatment cost of $52,834 for nal-iri/5FU and $1,809 for FOLFIRI.
These results in a real-world patient population demonstrate a similar PFS and OS for advanced pancreatic cancer patients treated with either nal-iri/5FU or FOLFIRI. In the absence of formal statistical testing, visual inspection of the time-to-event curves did not demonstrate a clear survival advantage for nal-iri/5FU. Although this was a small, single-center study, our survival outcomes were similar to those published in previous trials.1,5-7 In addition, patients experienced adverse effects with a similar frequency in both groups, which does not seem to support the hypothesis that the liposomal formulation of nal-iri spares healthy cells from exposure to irinotecan or its metabolites in a clinically meaningful way. FOLFIRI patients were more likely to need atropine, but this did not translate into a clear difference in the reported rates or severity of diarrhea.
The difference in overall toxicity management, with FOLFIRI patients more likely to have a dose reduction while nal-iri/5FU patients were more often delayed, was an interesting contrast that we hypothesize could be a result of oncology team familiarity with each regimen. Since FOLFIRI has been utilized for decades in many different GI malignancies, oncologists and pharmacists might be more comfortable determining an appropriate dose reduction might be for a given patient, whereas they may opt for a treatment delay for the newer and less-familiar nal-iri/5FU regimen.
The decision to select one treatment over another involves several factors, including efficacy and safety, and may also include considerations for cost or convenience. After FDA approval of nal-iri, no institutional protocols or guidelines encouraged providers to select one treatment over the other in any specific scenario. Therefore, the decision to treat with nal-iri/5FU or FOLFIRI at our institution was based on provider- and patient-specific factors and determined on a case-by-case basis.
This study did not demonstrate an obvious difference in either survival outcomes or adverse effect frequencies. Our cost analysis, with an acknowledgement of its inherent limitations for applicabil-ity to a health system with complex payment and reimbursement models, undoubtedly shows treatment with nal-iri/5FU is significantly more expensive than treatment with FOLFIRI.
Together, these factors support consideration of FOLFIRI in place of nal-iri/5FU for the treatment of advanced pancreatic cancer. Of course, a well-powered randomized controlled trial would be able to more definitively answer the question of non-inferiority. The oncology clinician may at least take comfort that these data do not suggest we do our patients a disservice by electing for treatment with FOLFIRI for advanced pancreatic cancer, particularly when the costs of treatment are of concern.
REFERENCES
- Wang-Gillam A, Li CP, Bodoky G, et al.; NAPOLI-1 Study Group. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387(10018):545-5 5 7.
- Yoo C, Hwang JY, Kim JE, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer. 2009;101(10):1658-63.
- Kalra AV, Kim J, Klinz SG, et al. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res. 2014;74(23):7003-13.
- Tossey JC, Reardon J, VanDeusen JB, et al. Comparison of conventional versus liposomal irinotecan in combination with fluorouracil for advanced pancreatic cancer: a single-institution experience. Med Oncol. 2019;36(10):87.
- Glassman DC, Palmaira RL, Covington CM, et al. Nanoliposomal irinotecan with fluorouracil for the treatment of advanced pancreatic cancer, a single institution experience. BMC Cancer. 2018;18(1):693.
- Gebbia V, Maiello E, Giuliani F, et al. Irinotecan plus bolus/infusional 5-fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol. 2014;32(23):2423-9.
- Zaniboni A, Aitini E, Barni S, et al. FOLFIRI as second-line chemotherapy for advanced pancreatic cancer: a GISCAD multicenter phase II study. Cancer Chemother Pharmacol. 2012;69(6):1641-5.
Venetoclax use in AML: VIALE-A & VIALE-C Trial Updates
Sonal Agarwal, PharmD
Hematology Oncology Clinical Pharmacist
Yale New Haven Hospital
New Haven, CT
Molly Schiffer, PharmD BCOP
Hematology Oncology Clinical Pharmacist
Yale New Haven Hospital New Haven, CT
Among adult types of acute leukemia, acute myeloid leukemia (AML) is the most common and has the highest mortality rate with a 28.7% five-year relative survival. 1,2 AML is predominantly a malignancy that affects older adults who are at greater risk for complications and co-morbidities.2
Initial treatment management must take into consideration a patient’s performance status, comorbid conditions, cytogenetic and molecular mutations, and age. Intensive induction therapies with cytarabine and an anthracycline are typically offered to young, healthy patients.3 However, due to co-morbidities, older adults, many may not be candidates for intensive therapy.4 Therapeutic options for these individuals have historically included agents such as hypomethylating agents (HMAs), low dose cytarabine (LDAC), gemtuzumab, or best supportive care.4-7
Venetoclax (ABT-199) is a BH3 mimetic that has high selectivity for the B-cell lymphoma-2 (BCL-2) protein. BCL-2, an antiapop-totic protein, has appeared to be an effective target in AML in preclinical studies.8-9 Venetoclax was approved in November 2018 for the treatment of newly-diagnosed AML in adults who are age 75 years or older, or who have comorbidities that preclude use of intensive induction chemotherapy based on phase Ib and phase II studies assessing the combination of venetoclax with both HMAs and LDAC10,11. The recently published, confirmatory VIALE-A and VIALE-C phase III trials further establish the efficacy and safety of the azacitidine-venetoclax regimen and the LDAC-venetoclax regimen, respectively.
Azacitidine in Combination with Venetoclax: VIALE- A Trial12
VIALE-A study was a confirmatory phase III, multicenter, randomized, double-blind, placebo-controlled trial that evaluated efficacy and safety of venetoclax in combination with azacitidine. All newly diagnosed AML patients who were older than 18 years of age and ineligible to receive intensive chemotherapy regimen were included. Patients were excluded if they had received prior hypomethylating agents, venetoclax, or chemotherapy for myelodysplastic syndrome (MDS), or had favorable cytogenetic risk. Patients were randomized 2:1 to receive azacitidine-venetoclax or azacitidine-placebo (control group) until disease progression or unacceptable toxicity.
The primary endpoint was overall survival (OS), and important secondary endpoints evaluated were event free survival (EFS), composite complete remission (complete remission and complete remission with incomplete hematologic recovery), complete remission, and time to first response. Treatment-related adverse events were assessed in all patients who received at least one dose of therapy.
From February 2017 through May 2019, 431 patients were included, out of which 286 patients were randomized to azacitidine plus venetoclax arm and 145 to azacitidine plus placebo arm. All patients received azacitidine 75 mg/m2 subcutaneously or intrave-nously on days 1 through 7, along with venetoclax target dose of 400 mg or matching placebo oral daily in 28-day cycles. Notably, the recommended venetoclax dose adjustment in combination with a strong CYP3A inhibitor differs between the VIALE-A protocol and the package insert.13 In the AML population, the FDA-approved recommendation is to titrate venetoclax to a maximum of 100mg daily when co-administered with a CYP3A inhibitor; however, the VIALE-A trial took a more conservative approach and utilizes a maximum dose of 50 mg daily. Seventy-five percent of patients had de novo AML and 25% had secondary AML that included history of MDS, chronic myelomonocytic leukemia (CMML), or therapy-related AML. The median duration of follow up was 20.7 months.
The median overall survival was significantly improved with azacitidine-venetoclax (14.7 months vs 9.6 months; hazard ratio for death [HR], 0.66; P<0.001). Composite complete remission achieved was significantly better in the treatment arm (66.4% vs 28.3%; P<0.001); and the complete remission was higher in the treatment arm (36.7% vs 17.9%; P<0.001). Median event-free survival was also significantly improved in the treatment arm (9.8 months vs 7 months; HR 0.63; P<0.001). Time to first response was more rapid in the combination group (1.3 months vs 2.8 months).
For safety analysis, 427 patients were included, out of which 283 patients were in the azacitidine-venetoclax arm and 144 were in the control group. The median number of treatment cycles received in each arm were 7.0 and 4.5 respectively. At least one serious adverse event was reported in 83% of patients in the treatment arm and 73% of patients in the control arm. The most common grade 3 or higher hematologic toxicities in the combination arm versus azacitidine-placebo arm were neutropenia (42% vs 28%), thrombocytopenia (45% vs 38%), febrile neutropenia (42% vs 19%), and anemia (26% vs 20%). Tumor lysis syndrome was seen in 1% of patients during the ramp-up period of venetoclax in combination arm.
Low-Dose Cytarabine in Combination with Venetoclax: VIALE-C Trial 14,15
VIALE-C is a similar confirmatory phase III, multicenter, double-blind, placebo-controlled clinical trial that evaluated the efficacy and safety of venetoclax in combination with LDAC. Adults 18 years or older with newly diagnosed AML and ineligible for intensive chemotherapy were randomized 2:1 to receive LDAC-venetoclax or LDAC-placebo (control group). Patients were considered ineligible for intensive chemotherapy if either they were ≥75 years of age or 18 to 74 years old with at least one criterion that was associated with lack of fitness for intensive chemotherapy regimen. Patients who had previous exposure to hypomethylating agents were included in the study. Excluded patients include those who received prior treatment with cytarabine for MDS, received strong or moderate CYP3A4 inducers seven days prior to the first dose of venetoclax, had known central nervous system involvement, or had WBC >25 x 109/L.
The VIALE-C study took a similarly conservative approach to venetoclax dose adjustments as the VIALE-A study and also had differing recommendations when compared to the package insert.12,13 Primary endpoint was overall survival, and key secondary endpoints included composite complete response and event-free survival.
Overall, 211 patients were enrolled between May 2017 and November 2018; 143 were randomized to LDAC-venetoclax arm and 68 patients were randomized to LDAC-placebo (control) arm. Patients received low-dose cytarabine 20 mg/m2 subcutaneously on days 1 through 10, along with venetoclax target dose of 600 mg (start at 100 mg on day 1 and escalated over 4 days) or matching placebo oral daily in 28-day cycles. Approximately 60% of patients were ≥75 years of age, 38% of patients had secondary AML, and 20% received prior hypomethylating agents.
At planned primary analysis, median overall survival was not significantly higher in the combination arm (7.2 months vs 4.1 months). LDAC-venetoclax arm showed reduction in the risk of death by 25% (HR 0.75; P=0.11) but was not statistically significant. At the six-month update after additional follow up, LDAC-venetoclax reduced the risk of death by 30% and median overall survival was 8.4 months vs 4.1 months in favor of the combination arm (P=0.04). Composite complete response were 48% and 13% in combination arm and control arm, respectively. Median event-free survival significantly improved in the treatment arm (4.7 months vs 2.0 month, P=0.002). A more rapid response was also seen with the combination arm, with a composite complete remission of 34% vs 3% seen after the first cycle in the combination arm and placebo arm, respectively.
For safety analysis, a total of 210 patients (142 in combination arm and 68 in placebo arm) were evaluated. Serious adverse events reported were similar in both arms (65% vs 62%). Most common grade 3 or higher adverse events seen in both arms were neutropenia (47% vs 16%), thrombocytopenia (45% vs 37%), and febrile neutropenia (32% vs 29%). Adverse events leading to death (23% vs 21%) or treatment discontinuation (25% vs 24%) were similar in both arms.
Conclusion
The VIALE-A and VIALE-C trials confirmed previous phase I and II efficacy and safety results and showed the addition of venetoclax to azacitidine or LDAC improved overall survival and resulted in higher composite complete remission and faster response with a tolerable safety profile. This redefined the first-line treatment approach for older patients with newly-diagnosed AML who are unfit to receive intensive induction chemotherapy.
REFERENCES
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2019;69:7-34.
- Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2016. Bethesda, MD: National Cancer Institute. 2019.
- Fernandez, HF, Sun Z, Yao X, et al. Anthracycline Dose Intensification in Acute Myeloid Leukemia. N Engl J Med. 2009;361:1249-1259.
- Medeiros B, Satram-Hoang S, Hurst D, et al. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol. 2015;94:1127-1138
- Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379-389.
- Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with 30% blasts. Blood. 2015;126(3):291-299.
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670-2677.
- Pan R, Hogdal LJ, Benito JM, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4(3):362–675.
- Konopleva M, Letai A. BCL-2 inhibition in AML: an unexpected bonus? Blood. 2018;132(10):1007–1012.
- DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216-28.
- Wei AH, Strickland SA Jr., Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277-1284.
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020;383(7):617-629.
- Venclexta [Package Insert] South San Francisco, CA: Genentech USA, Inc, 2020
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137-2145.
- Wei AH, Montesinos P, Ivanov V, et al. A phase III study of venetoclax plus low-dose cytarabine in previously untreated older patients with acute myeloid leukemia (VIALE-C): A six-month update. J Clin Oncol.2020;38(15):7511-7511
