HOPA Publications Committee
Brandy Strickland Snyder, PharmD BCOP, Chair
Bonnie Labdi, PharmD RPh, Vice Chair
Susanne E. Liewer, PharmD BCOP, Board Liaison
Carrie L. Barnhart, PharmD
Lisa R. Biondo, PharmD BCPS
Jennifer L. Davis, PharmD
Christine Gegeckas, RPh BCOP
Ashley Glode, PharmD BCOP
Megan Lynn Hames, PharmD BCPS
David Reeves, PharmD BCOP
Alexandra Shillingburg, PharmD
Candice Wenzell, PharmD
Board Update

Niesha Griffith, RPh MS FASHP, HOPA President
Well, it certainly has been an interesting winter! If nothing else, we have all learned a lot about weather systems caused by the “polar vortex” and the “pineapple express.” Personally, I have had just about enough of the snow and cold; however, I know we have been spared here in Ohio compared with those on the East Coast or even those in the South, who are just not prepared to deal with this kind of weather.
I was not pleased to hear that Punxsutawney Phil, the world’s most famous furry forecaster, popped out of his burrow and predicted 6 more weeks of winter. I was, however, very pleased to hear that Buckeye Chuck (no relation to Phil) did not see his shadow. I do realize that this is not a scientific exercise, but Chuck only lives an hour away from Columbus, so I have decided he probably knows best regarding what’s in store for us Buckeyes. After dealing with below-zero temperatures and weekly snow storms, I think we are all looking for some good news wherever we can find it!
HOPA Annual Meeting
Speaking of good news, our annual meeting is less than a month away! This year, our conference is expected to draw more than 800 pharmacists from all over the world—nearly half of our entire HOPA membership. Our conference educational programs have been developed with an eye toward providing a variety of cutting-edge sessions that serve the needs of both new and seasoned oncology pharmacists.
We will hold two preconference workshops: Oncology 201, covering topics such as bladder, uterine, and thyroid cancer; and a program for oncology residency and preceptor program development.
We will kick off the conference on Wednesday afternoon with the John G. Kuhn Keynote Lecture. This year features our very own John Kuhn (a founding member and our first HOPA president), and Kevin E. O’Connor, who is an author, executive coach, and professional speaker. John and Kevin will reflect on the current state of oncology pharmacy, how far HOPA has come as an organization, and what the future may hold for both our profession and the organization.
Many of our nation’s leading experts in hematology/oncology pharmacy will share their knowledge via educational and interactive sessions featuring a wide range of topics, including new and emerging therapies, controversies in care, and clinical pearls. New this year are two research sessions: developing and submitting a high-quality research proposal and conducting research on clinical service development and evaluation. Breakout sessions will address chemotherapy dosing in obese patients, updates on closed-system transfer devices, and the use of chemotherapy during pregnancy, among others. Our lobbyists from Drinker Biddle & Reath will provide an update on legislative issues affecting HOPA and a review of our health policy priorities and activities.
Several networking events will offer attendees an opportunity to expand their professional contacts. Our exhibit hall will be filled with the premier providers of pharmaceutical products, devices, and delivery systems. As always, our members will have an opportunity to review the latest in completed research during the poster sessions. All conference information, including session descriptions and a list of exhibitors, is available at Conference Web Central on the HOPA website. Be sure to check it out!
Scope of Hematology/Oncology Pharmacy Practice
In a previous board update, I mentioned that the Scope of Hematology/Oncology Pharmacy Practice was complete and available on our website. Since that time, an abbreviated version has been accepted for publication in the Journal of Oncology Pharmacy. I want to offer a big thank you and congratulations to Lisa Holle and Laura Michaud for spearheading this publication and producing a document to increase awareness of our profession in the hematology/oncology community.
Industry Relations Council
In the spirit of good news, I am happy to report that we now have 14 Industry Relations Council (IRC) members. We had five new members sign on since January (Bayer, Boehringer-Ingelheim, Helsinn Therapeutics, Lilly Oncology, and Seattle Genetics). Thank you to all of the IRC members for their continued support of HOPA.
Miscellaneous Updates
In late November, a work group met to begin developing a HOPA guideline that will address medication therapy management for oral anticancer agents. In February, the work group editors submitted their outline, references, and presentations to the medical writer to compose the first draft. Members will have an opportunity to review and comment on the guideline in spring/summer 2014. The HOPA Investigational Drug Service Best Practice Standards is now in production and will be available for member comment in April 2014.
HOPA is in the final stages of completing two issues briefs, which are also expected to be released as early as this spring. One will focus on pain management in cancer patients, while the other identifies measures for preventing counterfeit medications from entering our secure supply chains.
The Volunteer Activity Center is open until March 31. Please sign in and let us know whether you would be interested in participating in any HOPA committees.
The board is suggesting a number of bylaws changes to support our new leadership development plans. Among these proposals will be a name change for the Nomination and Awards Committee and a term change for board members. Watch for the notification of the revisions to be posted on our website for a 45-day member comment period.
HOPA 10th Anniversary Gala
Last, but certainly not least, to recognize the founding of HOPA 10 years ago, we will host the HOPA 10th Anniversary Gala on the evening of Friday, March 28, at The Chicory, which is just a 5-minute walk from the conference hotel. The event will feature a buffet dinner of Creole cuisine, an open bar, and live entertainment provided by some of New Orleans’ finest jazz musicians. During this festive evening, we will raise money for the HOPA Research Fund and recognize those individuals who were instrumental in the founding and shaping of this great organization. Tickets are available online at hoparx.org or by calling 1.877.HOPARX1.
As this is my last board update, I want to take this opportunity to thank the HOPA Board, staff, IRC, and all of you—our dedicated members—for your help and support during the past year. I am excited about all of our accomplishments and growth. We now have
2,093 members, more than 150 members than this time last year!
I look forward to supporting HOPA’s efforts in my final year on the board as past president and in the future as an active member. I sincerely hope to see many of you in New Orleans for the meeting.
Here’s looking forward to a memorable 10th anniversary, successful meeting, and warm spring!
Palliative Care and Hospice: A Review for the Hematology/ Oncology Practitioner
Stephanie Abel, PharmD
PGY-1 Pharmacy Practice Resident
Medical University of South Carolina, Charleston, SC
Palliative care and hospice currently are buzzwords in the oncology community. The American Society of Clinical Oncology (ASCO) published a Clinical Consensus Statement in 2012 urging oncologists to consider palliative care at the time of diagnosis of metastatic cancer or at any time in those with a high symptom burden.1 The National Comprehensive Cancer Network (NCCN) Palliative Care Guidelines suggest that palliative care begin at cancer diagnosis in concordance with disease-directed, life-prolonging therapies.2
The utilization of palliative care and hospice services is increasing and will likely continue as patient and caregiver outcomes improve, satisfaction with these services increases, and proposed economic benefits are realized.1 This article defines palliative and hospice care, summarizes recent evidence regarding the benefit of these services, identifies symptoms that palliative care addresses, and summarizes the pharmacist’s role in providing palliative care services.
Definitions
Palliative care is comprehensive patient and family care focusing on the relief of distressing symptoms of a chronic illness.2 This is accomplished by incorporating psychosocial and spiritual care individualized to patients’ and families’ needs, values, beliefs, and cultures. Goals of palliative care include anticipating, preventing, and reducing the suffering of the patient and family, in addition to supporting the best quality of life throughout the course of the disease.2
Hospice care focuses on the patient’s quality of life as opposed to length of life. The goal of hospice care is to provide humane and compassionate care for patients during the late phases of an incurable disease so that they may live as fully and comfortably as possible.3 Hospice care and palliative care are very similar in that they both seek to provide the patient with the best possible quality of life. However, patients must have an estimated prognosis of 6 months or less to qualify for hospice care services in the United States. In essence, all hospice care is palliative care but not all palliative care is hospice. Palliative care can be provided during all stages of chronic disease and should not be equated to end-of-life (EOL) care. If the patient lives longer than 6 months, they may still participate in hospice care as long as their prognosis does not extend beyond 6 months. The philosophy of hospice is to accept death as the final stage of life. Hospice services neither hasten nor postpone death and promote treating the patient as a whole person rather than just treating a disease.
Overview of Recent Evidence
Temel and colleagues4 conducted a study of 151 adult patients with newly diagnosed metastatic non-small-cell lung cancer (NSCLC) who were randomized to receive early palliative care (PC) in addition to standard oncology care or to solely receive standard oncology care. Patients were recruited from a single outpatient clinic in Massachusetts. The intervention arm received a baseline PC assessment and an outpatient follow-up visit at least monthly with a multidisciplinary PC team. The primary outcome was change in quality of life (QOL) at 12 weeks based on the Trial Outcome Index (TOI), which consisted of the sum of scores from the Lung Cancer Sub-scale (LCS) and the Functional Assessment of Cancer Therapy-Lung (FACT-L). The FACT-L evaluates several aspects of QOL and the LCS is a subscale of the FACT-L that evaluates seven symptoms specific to lung cancer. The FACT-L scale is validated and has been used extensively for QOL assessment in patients with lung cancer.5 Secondary outcomes included mood assessments and incidence of aggressive EOL care defined as chemotherapy within 14 days of death, lack of hospice care, or hospice admission ≤3 days before death.
When comparing QOL, the PC intervention group had significantly higher scores in the FACT-L, TOI, and LCS (p = .03, .009, and .04, respectively). The PC intervention group had fewer depressive symptoms as measured by the Hospital Anxiety and Depression Scale (HADS) and Patient Health Questionnaire 9 (PHQ-9; p = .01). HADS and PHQ-9 are both validated scales and are commonly used to measure the outcomes assessed in this study.6,7 At the time of analysis of EOL care, 105 patients (70%) had died. Aggressive EOL care was more common in the standard oncology care group compared with the PC group (54% versus 33%, p = .05). Although patients in the PC group had less aggressive EOL care, the patients in this arm survived 2.7 months longer than those in the standard oncology care group (p = .02).4
Gade and colleagues8 at Kaiser Permanente randomly assigned 512 seriously ill patients receiving care in the hospital at three sites within the United States to receive either usual care (UC) or usual care plus an interdisciplinary palliative care service (IPCS). The percentage of patients with a cancer diagnosis was 27.3% and 34.4% in the IPCS and UC groups, respectively. The primary outcomes were to assess patient satisfaction, clinical outcomes assessed by overall survival, and cost of care for 6 months after hospital discharge. The IPCS treatment arm reported greater satisfaction with their care experience (p = .04). There was no statistically significant difference between groups in median survival from study enrollment and death during the study period (p =.08 for both measures). The total mean health costs were $6,766 lower in the IPCS group (p < .001). In addition, patients in the IPCS arm had significantly fewer intensive care unit readmissions (IPCS: n = 12, usual care: n = 21, p = .04).
Palliative care models are not uniform in the literature, and although many models have been assessed, a benefit has been shown despite a lack of standardization. In addition, no studies to date have shown harm in any form to patients from a palliative care or hospice care intervention. Many national and international organizations have adopted a positive stance on the use of palliative care and hospice care in chronic illness and at the end of life based on the benefits observed in clinical trials.1
Symptoms Addressed by Palliative Care
There are several symptoms (Table 1) that patients should be assessed for during each visit that may have an impact on their QOL. Many of these symptoms may be treated with both nonpharmacologic and pharmacologic measures and can be valuable interventions made by the pharmacist on the team. The NCCN and World Health Organization (WHO) offer thorough and complete resources that provide guidance on treatment options to address these symptoms.
Role of the Pharmacist
Medication therapy management is a large part of symptom management in palliative care, and, therefore, pharmacists have the potential to make a great impact on this area of care. Some traditional aspects that apply to most areas of pharmacy practice apply in palliative care, including assessing medication appropriateness, reviewing medication profiles, counseling patients and caregivers, and providing drug information. Patients who are approaching the end of life may not tolerate adverse drug reactions and have an increased risk of iatrogenic complications. In addition, medications that prove to be ineffective for the patient need to be identified and modified quickly because time and goals of care may be of the essence.10 Pharmacists should keep in mind the patient’s current goals of care, condition, tolerance of current regimen, and financial situation when performing a medication profile review. Medication administration issues may arise and the pharmacist can provide valuable input as to methods of administration, compounding options, and appropriate agent selection individualized to patient-specific circumstances.
Patient and caregiver education is of the utmost importance in palliative care. Medication regimens are only as effective as the patient’s and caregiver’s understanding of and adherence to the therapeutic plan. A patient’s concern for symptom management may be clouded by concern about addiction, side effects, the social stigma associated with taking many medications, or financial barriers.10 Taking the time to understand the potential barriers to adherence, educating the patient and caregiver, and providing expertise to help navigate these medication-related issues can influence the success of a medication regimen.
In addition to patient education, the pharmacist can provide invaluable education to the healthcare team. As noted above, patient barriers to effective symptom management exist. Similarly, barriers to effective symptom management and pain control are present within the health-care system as well as within the healthcare team. Healthcare professionals may have inadequate knowledge to assess and manage pain, fear of patient addiction or tolerance, concern for side effects of analgesics, or concern about regulation of controlled substances. Barriers to effective pain management also exist within the healthcare system. Insurance companies may inadequately reimburse for pain assessment and treatment and may not cover the best pain management agents for the patient. The medications also may be too expensive even if the patient has insurance and therefore may not be feasible options. Treatment availability may be limited or access may be restricted. Not all of these barriers can be addressed immediately, but keeping them in mind while providing patient care may help avoid potential problems.11 The pharmacist is well suited to help identify and address these issues to improve patient outcomes.

Conclusion
Palliative care focuses on treating the whole person and not just the disease state. Hospice care utilizes the principles of palliative care in a patient population with a prognosis of less than 6 months to live. Palliative care services have been shown in the literature to provide benefit to patients by improving QOL, providing less aggressive EOL care, decreasing healthcare costs, and prolonging survival. The pharmacist can play an integral role on a palliative care team or a general oncology team as an advocate for palliative care. In addition to general pharmacy practice services, the pharmacist can educate the patient, caregiver, and healthcare team about medication options, adherence, and barriers to adherence. The pharmacist can help assess symptoms at each encounter, assess efficacy of the current regimen, and make pharmacologic and nonpharmacologic recommendations throughout the course of palliative care treatment.
References
1. Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012 Mar 10;30(8):880-887.
2. National Comprehensive Cancer Network. NCCN clinical practice guidelines: palliative care v.2.2013.
3. What does hospice care provide? American Cancer Society website. www.cancer.org/treatment/findingandpayingfortreatment/choosingyourtreatmentteam/hospicecare/hospice-care-services. Accessed September 24, 2013.
4. Temel JS, Greer JA, Muzikansky A, et al. Early palliative carefor patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010 Aug 19;363(8):733-742.
5. Functional Assessment of Cancer Therapy-Trial Outcome Index (for Lung Cancer)—(FACT-TOI) Online Document. Radiation Therapy Oncology Group website. www.rtog.org/LinkClick.aspx?fileticket=JTr_jD15mvw%3D&tabid=118. Accessed December 18, 2013.
6. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002 Feb;52(2):69-77.
7. Instructions for Patient Health Questionnaire (PHQ) and GAD-7 Measures. PHQ Screeners website. www.phqscreeners.com/instructions/instructions.pdf. Accessed December 18, 2013.
8. Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008 Mar;11(2):180-90.
9. Palliative care: Symptom management and end-of-life care Web document. World Health Organization website. www.who.int/ hiv/pub/imai/genericpalliativecare082004.pdf. Accessed November 15, 2013.
10. Cortis LJ, McKinnon RA, Anderson C. Palliative care is everyone’s business, including pharmacists. Am J Pharm Educ. 2013 Mar 12;77(2):21.
11. Pain (PDQ®): Supportive care—Health Professional Information [NCI]. University of Wisconsin Health website. www.uwhealth.org/health/topic/nci/pain-pdq-supportive-care-health-professional-information-nci/ncicdr0000062738.html. Accessed October 26, 2013.
Food and Drug Administration Drug Approval Processes: Speedier Access to New Treatments
Caitlin N. Swann, PharmD
PGY2 Hematology/Oncology Pharmacy Resident
Cleveland Clinic, Cleveland, OH
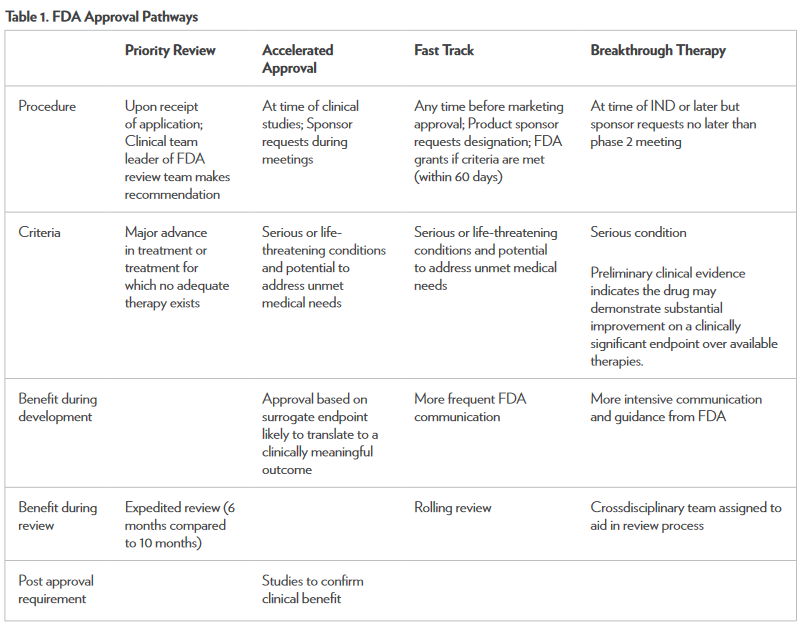
Recently, there have been changes in the U.S. Food and Drug Administration’s (FDA’s) drug approval process to make important therapies available to patients sooner than ever before.1 This article reviews the various pathways developed by the FDA to expedite the drug approval process. Specifically, priority review, accelerated approval, fast-track designation, and the breakthrough therapy designation will be discussed along with examples of drugs that have gone through these programs.
Priority Review Versus Standard Review
To be eligible for priority review, a drug must treat a serious condition and provide a significant improvement in safety or effectiveness compared to standard treatment.1 A major benefit of this designation is that the FDA will review and make a decision on the drug approval application within 6 months, compared with 10 months under the standard review process. Those drugs granted priority review will also receive additional attention and resources from the FDA during the evaluation phase.
Accelerated Approval
The advantage of undergoing accelerated approval is that a drug may be approved based on a surrogate endpoint that is likely to predict a clinical benefit without having to demonstrate the clinical benefit itself.1 With accelerated approval, drugs can be approved much faster than the time it would take to demonstrate the drug’s impact on morbidity or mortality. To qualify for accelerated review, the drug must be used for a serious or life-threatening condition for which acceptable treatments are lacking. In conjunction with accelerated approval, the FDA requires the sponsors to agree to conduct post approval studies to verify that a clinical benefit has been demonstrated. If the study results confirm a clinical benefit, the FDA will convert the accelerated approval to traditional approval. If these studies fail to demonstrate a clinical benefit, the FDA may withdraw their approval of the agent, as was the case with ponatinib.
Ponatinib (Iclusig®), which is a tyrosine kinase inhibitor indicated for the treatment of chronic myeloid leukemia (CML) and Philadelphia positive (Ph+) acute lymphocytic leukemia (ALL) resistant or intolerant to other tyrosine kinase inhibitors, was approved in December 2012.2 During the initial phase 2 approval trial of this agent, a complete cytogenetic response was demonstrated in 46% of patients, while a major cytogenetic response was shown in 56% of patients, and a major molecular response was observed in 34% of patients.3 In addition, a subset of patients with the T315I mutation demonstrated even greater benefits from the drug, with 70% achieving a major cytogenetic response.3 The presence of the T315I mutation confers resistance to all previously tested tyrosine kinase inhibitors; ponatinib was shown to be the exception. Approval of ponatinib would provide a much needed treatment option for patients with this particular genetic mutation.
The FDA granted accelerated approval of ponatinib with the understanding that additional studies would be performed to confirm its benefit on morbidity and mortality and to further evaluate its safety.4At the time of approval, the initial study results demonstrated a favor- able risk profile for ponatinib. Unfortunately, as the data matured, the frequency of serious and life-threatening blood clots and severe narrowing of blood vessels increased from 9% in the initial reports to at least 27% in the most recent results.5 The FDA suspended the marketing and sales of ponatinib on October 31, 2013. During this time, the FDA analyzed the potential benefit in the subset of patients in whom this agent may still possess a favorable risk/ benefit ratio, specifically those with the T315I mutation. Ponatinib was not commercially available but could be obtained through a patient-specific investigational new drug (IND) or expanded access registry program. In December 2013 the FDA announced the reauthorization of marketing and sales of the drug with several new safety measures in place. A revised Risk Evaluation and Mitigation Strategy (REMS) highlights the cardiovascular risks associated with ponatinib as well as the new indications for its use. The indications are now limited to specific groups of patients: adults with T315I-positive CML or Ph+ ALL, and adults with chronic, accelerated, or blast-phase chronic myeloid leukemia or Ph+ ALL when no other tyrosine kinase inhibitor therapy is indicated. The commercial distribution of the drug resumed in mid-January 2014. Ponatinib is an example of how the accelerated approval process is meant to provide patients access to potentially life-saving treatments as soon as possible, while at the same time protecting the public from situations in which the actual risks outweigh the perceived benefits of treatment.
Fast Track Designation
The fast track designation also promotes expedited approval for drugs.6 In this program, sponsors of the drug will have frequent interactions with the FDA to discuss the drug’s development plan and field any questions about the process. If the FDA grants a drug fast track designation status, the sponsor may file certain portions of the marketing application before submitting the complete application in a process known as rolling review. Fast track designation may be requested at any stage during drug development. To qualify for the fast track designation, the drug must be intended for the treatment of a serious or life-threatening disease or condition and it must demonstrate the potential to address unmet medical needs for the intended disease or condition. An unmet medical need exists when available therapy does not address the treatment or diagnosis of a condition. The defining criteria or type of information needed to prove the drug addresses an unmet medical need depend on how far along in the process this expedited status is requested. For example, in nonclinical models, the rationale behind the mechanism of action or other pharmacologic data may be considered sufficient if the drug is early in the development phase. In contrast, if the drug is in the later stages of development and there are clinical data available, then this clinical data should be used to justify the potential to address an unmet need.
Breakthrough Therapy Designation
Breakthrough therapy designation is the newest FDA drug approval category, which was signed into law in 2012.6 This designation provides all of the same features that the fast track designation does but also allows for added guidance from the FDA. For drugs granted this designation, the FDA forms a multidisciplinary team that meets with the sponsor of the drug to provide advice in designing trials that will gather the necessary data efficiently to expedite the commercial approval of the drugs. In addition, the FDA will assign a senior manager to the approval application, along with a crossdisciplinary project lead from their review team to act as a liaison with the sponsor throughout the development process.
For a drug to receive the breakthrough therapy designation, the drug must meet certain criteria.6 It must be used to treat a serious condition or disease associated with morbidity and mortality, and it must have the potential to have a substantial impact on day-to-day functioning. In contrast to the fast track designation, this designation does not require an absence of available treatments for the disease or condition. Ideally, this designation can be used for drugs that demonstrate substantial improvements over existing therapies when measured using one or more clinically significant endpoints. These endpoints refer to those that measure effects on morbidity or mortality or on the presence and severity of symptoms caused by the disease. This is in contrast to the various forms of nonclinical information (e.g., theoretical or mechanistic rationale, early nonclinical data) required for fast track designation.
This new program is beginning to demonstrate an impact on drug development. In January 2013, the first two drugs to receive breakthrough designations were announced.1 Both designations were awarded to Vertex Pharmaceuticals, which was seeking to expand the use of their cystic fibrosis drug ivacaftor (Kalydeco®). As of November 8, 2013, 92 requests for breakthrough designations had been made, of which 30 were granted, 47 were denied, and 15 were still under review. The first drug with the breakthrough designation to be granted FDA approval was obinutuzumab (Gazyva®). It was approved on November 1, 2013, for use in combination with chlorambucil in patients with treatment-naïve chronic lymphocytic leukemia (CLL).7 The initial study that led to FDA approval of this drug was a phase 3, open-label study of 356 patients with previously untreated CLL.7,8 The study reported improvements in the primary endpoint of progression-free survival, which was 23 months with obinutuzumab plus chlorambucil versus 11.1 months with chlorambucil alone (p < .0001). The overall response rate was increased in the combination arm when compared with chlorambucil alone (76% versus 32.1%). Last, the median duration of response was 15.2 months versus 3.5 months, favoring the combination arm. The most common grade 3 or 4 adverse events for patients who received obinutuzumab in combination with chlorambucil compared with chlorambucil alone were infusion-related reactions during the first infusion (21% versus 0%), thrombocytopenia (11% versus 3%), and neutropenia (34% versus 16%), although the higher incidence of neutropenia did not correspond with an increased rate of infections in the obinutuzumab plus chlorambucil arm.
Following obinutuzumab’s approval, another drug received FDA approval under the accelerated approval program after being granted breakthrough therapy designation. Ibrutinib (Imbruvica®) received FDA approval on November 13, 2013, for patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.9 The drug made it through FDA review in just 4 months. Accelerated approval was granted based on the results of a phase 2 study that enrolled 111 previously treated patients with MCL.9,10 The primary endpoint was the overall response rate, with 66% of patients demonstrating this. Furthermore, 17% of patients achieved a complete response and 49% of patients achieved a partial response. The median duration of response was 17.5 months. Bleeding events, including bruising of any grade, occurred in 48% of patients, and 5% of these were grade 3 or higher. Treatment- emergent grades 3 or 4 cytopenias occurred in 41% of patients and 25% had grades 3 or 4 infection. As a condition of accelerated approval, the FDA required that the sponsor submit 24-month follow-up data for all patients in this single-arm trial. The sponsor must also submit the results of a randomized controlled trial comparing ibrutinib in combination with bendamustine plus rituximab to bendamustine plus rituximab alone in patients with newly diagnosed MCL.
Summary
There are several FDA programs available to expedite the process of drug development and review to make potentially life-saving treatment options available to patients sooner.1,6 Along with the benefits of these programs, there are limitations. It is possible for a drug to make it to market before it has demonstrated safety and effectiveness. According to the FDA, there are stringent measures in place that require aggressive review from multidisciplinary teams representing both the FDA and the drug sponsor.6 The key component necessary for all of these programs is effective and timely communication between the sponsor and the FDA. The benefits of these programs have been demonstrated with the expedited approvals of drugs such as obinutuzumab and ponatinib, which were both made available to patients in a relatively short time. Table 1 summarizes the unique aspects of each approval pathway.3 Any healthcare professional can and should report adverse effects caused by any medication—new or old—through the MedWatch section on the FDA’s website.11 The new FDA approval pathways have the potential to greatly impact the treatment of cancer. Drugs are being approved faster than ever, meeting the needs of many cancer patients.
References
1. Fast track, breakthrough therapy, accelerated approval and priority review. Expediting availability of new drugs for patients with serious conditions. U.S. Food and Drug Administration website. www.fda.gov/forconsumers/ byaudience/forpatientadvocates/speedingaccesstoimportantnewtherapies/ucm128291.htm. Updated June 26, 2013. Accessed December 13, 2013.
2. Iclusig™ (ponatinib) [package insert]. Cambridge, MA: ARIAD, Inc; December 2012.
3. Cortes E, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome–positive leukemias. N Engl J Med. 2013;369:1784-96.
4. Approved drugs: Ponatinib. U.S. Food and Drug Administration website. www.fda.gov/ Drugs/InformationOnDrugs/ApprovedDrugs/ucm332368.htm. Updated December 17, 2012. Accessed December 13, 2013.
5. FDA drug safety communication. U.S. Food and Drug Administration website. www.fda.gov/Drugs/ DrugSafety/ucm379554.htm. Updated January 7th, 2014. Accessed January 10, 2014.
6. Guidance for industry expedited programs for serious conditions–drugs and biologics. U.S. Food and Drug Administration website. www.fda.gov/downloads/ Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM358301.pdf. Updated June 2013. Accessed December 13, 2013.
7. Gazyva® (obinutuzumab) [package insert]. South San Francisco, CA: Genentech, Inc; November 2013.
8. Goede V, Fischer K, Humphrey K, et al. Obinutuzumab (GA101) plus chlorambucil (Clb) or rituximab (R) plus Clb versus Clb alone in patients with chronic lymphocytic leukemia (CLL) and preexisting medical conditions (comorbidities): Final stage 2 results of the CLL11 (BO21004) phase III trial [abstract]. Blood. 2013;122:6
9. Approved drugs: Ibrutinib. U.S. Food and Drug Administration website. Available at: ww.fda.gov/ Drugs/InformationOnDrugs/ApprovedDrugs/ucm374857.htm. Updated November 13, 2013. Accessed December 13, 2013.
10. Wang ML, Rule SR, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507-16.
11. Reporting serious problems to FDA. U.S. Food and Drug Administration website. www.fda.gov/ Safety/MedWatch/HowToReport/default.htm. Updated August 23, 2013. Accessed December 30, 2013.
HOPA Weighs in on FDA Proposal to Reschedule Hydrocodone Combination Products
Jordan Wildermuth, MSW, Health Policy and Advocacy Manager
In October, Janet Woodcock, MD, director of the Center for Drug Evaluation and Research, issued a press release on behalf of the U.S. Food and Drug Administration (FDA) stating its intent to submit a formal recommendation to the U.S. Department of Health and Human Services (HHS) to reclassify hydrocodone combination products from Schedule III to Schedule II. The decision comes after years of concern by the FDA regarding the abuse and misuse of opioid products. After the recommendation is reviewed by HHS, a final decision on the appropriate scheduling of hydrocodone combination products will be made by the Drug Enforcement Agency.
HOPA’s pain management workgroup convened in September 2013 to evaluate requests for HOPA’s support of issues related to access to pain medication for cancer patients and respond to legislative, regulatory, and industry changes and practices to ensure the responsible use of pain medications while maintaining access to pain medications for cancer patients. The workgroup, with the support of the Health Policy Committee, concluded that reclassifying hydrocodone combination products would negatively affect the appropriate management of pain and the patient’s quality of life.
HOPA determined that two responses were appropriate for this issue. The first was an opportunity to sign on to a letter addressed to HHS submitted by the Pain Care Forum, a coalition of which HOPA is a member. The Pain Care Forum’s mission is to “balance the fundamental rights of patients and clinicians with the challenge of risk containment for opioid misuse, abuse, and addiction associated with medical prescribing and use of controlled substances.” The letter outlined a three-part proposal that included maintaining hydrocodone combination products in Schedule III, changing the limits on prescriptions for Schedule III medications so that a telephone prescription for hydrocodone-containing products would not exceed a 10-day supply, and limiting the total amount of medication available through the original prescription plus refills to no more than a 90-day supply.
The second response was to send a letter from HOPA to HHS to bring focus to the patient and, more specifically, how pain affects the cancer patient. Among other points discussed in HOPA’s letter, it was noted that until Schedule II narcotics are available through e-prescriptions, oncology patients, while battling cancer and the side effects of treatments, would have to travel to their physician’s office to obtain a hard-copy prescription. For patients living in rural areas, the nearest oncologist office may be several hours away from the patient’s home. HOPA stated that the rescheduling of hydrocodone combination products to Schedule II is likely to cause more access to care problems rather than solving the drug diversion/abuse problem.
For more information about HOPA’s advocacy activities, visit www.hoparx.org/health-policy.
Recalls and Safety Alerts from the FDA
Recalls
ForeCYTE Breast Health Test and Mammary Aspiration Cytol- ogy Test (MASCT)
Atossa Genetics Inc. initiated a voluntary recall to remove the ForeCYTE Breast Health Test and the Mammary Aspiration Specimen Cytology Test (MASCT) device from the market. Atossa is removing the ForeCYTE Breast Health Test and the MASCT device from the market to address U.S. Food and Drug Administration (FDA) concerns about the current instructions for use (IFU), certain promotional claims used to market these devices, and the need for FDA clearance for certain changes made to the nipple aspirate fluid specimen collection process identified in the current IFU. To date, Atossa is unaware of any adverse incidents or injuries associated with the use of the ForeCYTE Breast Health Test, the MASCT device, or the processing method currently identified in the IFU.
www.fda.gov/ Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm370784.htm
Specialty Medicine Compounding Pharmacy Certain Unexpired Compounded Sterile Products: Recall—Particulate Matter Found in Vials
Specialty Medicine Compounding Pharmacy is voluntarily recalling all lots of certain unexpired human and veterinary sterile products to the consumer level due to particulate matter found in vials of a compounded dextrose injection product dispensed to a local hospital. Further testing and analysis of the medication is being conducted. If there is microbial contamination in products intended to be sterile, patients are at risk for serious, potentially life-threatening infections. The recalled products were distributed to hospitals and consumers located only within Michigan from July 1, 2013, through October 19, 2013. No products were distributed out of state. For a detailed list of affected products visit
www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm371563.htm
MedStream Programmable Infusion Pump and Refill Kits by Codman & Shurtleff: Class 1 Recall—Drug Over Infusion
FDA and Codman & Shurtleff, Inc., notified healthcare professionals of the class 1 recall of MedStream Programmable Pump and MedStream Refill Kit due to air in the pump reservoir, which may release a higher dosage of drug than expected, leading to drug overdose. This product may cause serious adverse health consequences, including low blood pressure (hypotension), an abnormally slow heart rate (bradycardia), loss of consciousness, and death.
www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm371988.htm
Nature’s Pharmacy and Compounding Center Sterile Com- pounded Products: Recall—Lack of Sterility Assurance
Nature’s Pharmacy and Compounding Center of Asheville, NC, is voluntarily recalling all lots of sterile products compounded by the pharmacy that are not expired to the consumer level. The product will be in the form of an injectable drug or an eye drop. The recall is being initiated due to concerns associated with quality control procedures that were observed during a recent FDA inspection and present a potential risk to sterility assurance.
www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm375412.htm
Hematology/Oncology Approvals and Safety Notifications
Inclusig (Ponatinib): Drug Safety Communication—Increased Reports of Serious Blood Clots in Arteries and Veins
The FDA is investigating an increased frequency of reports of serious and life-threatening blood clots and severe narrowing of blood vessels (arteries and veins) in patients taking the leukemia chemotherapy drug Iclusig (ponatinib). Data from clinical trials and postmarket adverse event reports show that serious adverse events have occurred in patients treated with Iclusig, including heart attacks resulting in death, worsening coronary artery disease, stroke, narrowing of large arteries of the brain, severe narrowing of blood vessels in the extremities, and urgent surgical procedures to restore blood flow. The FDA is actively working to further evaluate these adverse events and will notify the public when more information is available.
www.fda.gov/Safety/MedWatch/SafetyInformation/ SafetyAlerts-forHumanMedicalProducts/ucm370971.htm
ISMP Medication Safety Alert!
• November 14, 2013 (Volume 18, Issue 23): Management of overfill volume for chemotherapy is critical to ensure patients receive full doses of their medications. Healthcare organizations should develop standardized preparation methods for consistency.
• December 12, 2013 (Volume 18, Issue 25): There is confusion regarding the need to use 10-ml syringes for flushing and locking via vascular access devices, including implanted ports and peripherally inserted central catheter lines. Bard Access Systems is updating their information to state that, with the exception of a 1-ml prefilled syringe, and once patency is assured, medication administration with smaller diameter syringes can occur.
Changes in Safety Labeling
Arzerra (Ofatumumab) Injection
Changes to ofatumumab labeling include the following:
• Reactivation of hepatitis B virus (HBV) with some reports of fulminant hepatitis, hepatic failure, and death; all patients should be screened for HBV before starting treatment with ofatumumab.
• Tumor lysis syndrome can occur with ofatumumab.
• Progressive multifocal leukoencephalopathy (PML) resulting in death has occurred with ofatumumab. PML should be considered in patients with new onset of or changes in pre- existing neurological signs or symptoms.
www.fda.gov/ Safety/MedWatch/ SafetyInformation/ucm372685.htm
Tasigna (Nilotinib) Capsules
The following changes to nilotinib labeling have occurred:
• Avoid food 2 hours before and 1 hour after taking a dose of nilotinib.
• Electrolyte, calcium, and magnesium blood levels should be tested before initiating and periodically during treatment with nilotinib.
• Sudden deaths have occurred in 0.3% of chronic myeloid leukemia patients treated with nilotinib.
• Nilotinib can cause increases in serum lipase, and those patients with a history of pancreatitis may be at greater risk.
• Avoid administration with agents that are strong CYP3A4 inhibitors, or antiarrhythmic medications (including, but not limited to, amiodarone, disopyamide, procainamide, quinidine, and sotolol) and other medications that may prolong the QT interval. Therapy with nilotinib should be interrupted if treatment with any of these agents is started.
• Lower doses of nilotinib should be used in patients with mild to severe hepatic impairment due to increased nilotinib exposure.
www.fda.gov/ Safety/MedWatch/ SafetyInformation/ucm182234.htm
Gemcitabine Injection 38 mg/mL
The following change has been made to gemcitabine labeling:
Capillary leak syndrome (CLS) with severe consequences has been reported in patients receiving gemcitabine as a single agent or in combination with other chemotherapeutic agents. Discontinue gemcitabine if CLS develops during therapy.
www.fda.gov/ Safety/MedWatch/ SafetyInformation/ucm371309.htm
NEUPOGEN (Filgrastim)
The following changes have been made to filgrastim labeling:
• Thrombocytopenia has been reported in patients receiving NEUPOGEN. Platelet counts should be monitored closely.
• Information on Amgen’s Lactation Surveillance Program has been added.
• Splenomegaly has been added to the adverse reaction section according to postmarketing experience.
www.fda.gov/ Safety/MedWatch/ SafetyInformation/ucm219032.htm
Alimta (Pemetrexed for Injection)
The following change has been made to pemetrexed labeling:
Reports of immune mediated hemolytic anemia have occurred with pemetrexed used as a single agent or in combination with other agents.
www.fda.gov/ Safety/MedWatch/ SafetyInformation/ucm371329.htm
Gleevec (Imatinib Mesylate) Tablets
The following change has been made to imatinib mesylate labeling:
Gleevec can cause fetal harm when administered to a pregnant woman.
www.fda.gov/ Safety/MedWatch/ SafetyInformation/ucm255333.htm
Lupron Depot (Leuprolide Acetate for Depot Suspension) and Lupaneta Pack (Leuprolide Acetate for Depot Suspension; Nor- ethindrone Acetate Tablets)
The following labeling change has been made:
There have been postmarketing reports of convulsions in patients on leuprolide acetate therapy. These included patients with and without concurrent medications and comorbid conditions.
www.fda.gov/ Safety/MedWatch/ SafetyInformation/ucm374019.htm
Zaltrap (Ziv-Aflibercept) Injection
The following labeling change has been made to ziv-aflibercept:
Monitor proteinuria by urine dipstick analysis or urinary protein creatinine ratio (UPCR) for the development or worsening of proteinuria during Zaltrap therapy. Patients with a dipstick of =2+ for protein or a UPCR greater than 1 should undergo a 24-hour urine collection.
www.fda.gov/ Safety/MedWatch/ SafetyInformation/ucm374591.htm
Abraxane Injectable Suspension (Paclitaxel Protein-Bound Particles [Albumin-Bound])
The following labeling change has been made to Abraxane:
Cardiovascular: There have been reports of congestive heart failure, left ventricular dysfunction, and atrioventricular block with Abraxane. Most of the individuals were previously exposed to cardiotoxic drugs, such as anthracyclines, or had underlying cardiac history.
www.fda.gov/ Safety/MedWatch/ SafetyInformation/ucm359951.htm
Nexavar (Sorafenib)
The following labeling changes have been made to sorafenib:
• Osteonecrosis of the jaw.
• Impairment of thyroid-stimulating hormone suppression in differentiated thyroid carcinoma; Sorafenib impairs exogenous thyroid suppression.
www.fda.gov/ Safety/MedWatch/ SafetyInformation/ucm280363.htm
www.fda.gov/ Safety/MedWatch/ SafetyInformation/ucm319233.htm
Revlimid (Lenalidomide) Capsules
The following labeling change has been made to lenalidomide:
Lenalidomide should not be used to treat people who have chronic lymphocytic leukemia unless they are participants in a controlled clinical trial, due to increased mortality risk.
www.fda.gov/ Safety/MedWatch/ SafetyInformation/ucm299519.htm
Xalkori (Crizotinib) Capsules
The following labeling changes have occurred with crizotininb:
• Drug-induced hepatotoxicity with fatal outcome occurred in two (0.2%) of the 1,225 patients treated with crizotinib across three main clinical trials.
• Severe, life-threatening, or fatal interstitial lung disease/pneumonitis can occur in patients treated with crizotinib.
• QTc prolongation can occur in patients treated with crizotinib.
• Symptomatic bradycardia can occur in patients receiving crizotinib.
www.fda.gov/ Safety/MedWatch/ SafetyInformation/ucm295722.htm
Votrient (Pazopanib) Tablets
The following change has been made to the adverse reactions section of pazopanib labeling:
Arthralgia, muscle spasms
www.fda.gov/ Safety/MedWatch/ SafetyInformation/ucm303649.htm
Highlights from the 36th Annual CTRC-AACR San Antonio Breast Cancer Symposium (SABCS)
Bobbie Quach, PharmD Student
Meghana V. Trivedi, PharmD PhD BCOP
Assistant Professor
University of Houston College of Pharmacy, Houston, TX
Bisphosphonates in Breast Cancer
Adjuvant Bisphosphonate Reduces Bone Recurrence and Improves Survival in Postmenopausal Women with Early Breast Cancer1
During the past 15 years, numerous randomized controlled trials have shown conflicting clinical benefits in the use of adjuvant bisphosphonate therapy in breast cancer. To address this, a meta-analysis of 36 randomized controlled trials was conducted to compare bisphosphonate versus no bisphosphonate in the adjuvant setting. The primary outcomes were time to recurrence, time to first distant recurrence, and mortality. In all 17,709 women (pre- and postmenopausal) included in the study, a significant reduction in bone recurrence (10-year gain: 1.5%) was observed with the use of bisphosphonates. When the analysis was restricted to 11,306 postmenopausal women, the reduction in bone recurrence with bisphosphonate therapy was even higher (10-year gain: 2.9%). In the analysis of bone recurrence by menopausal status, use of adjuvant bisphosphonates significantly reduced bone recurrence in postmenopausal women (hazard ratio [HR] = 0.66) but not in premenopausal women (HR = 0.93). There was a significant delay in breast cancer recurrence and non-bone distant recurrence in postmenopausal women taking bisphosphonates; this difference was not seen when premenopausal women were also included in the analysis. In evaluating mortality in postmenopausal women, adjuvant bisphosphonate use significantly reduced breast cancer mortality (10-year gain: 3.1%) and all-cause mortality (10-year gain: 2.3%). Although the difference in breast cancer mortality was significant with bisphosphonates in all women, this was primarily driven by the majority of postmenopausal women. In summary, adjuvant bisphosphonates in postmenopausal women significantly reduced the risk of bone recurrence (risk reduction of 34%) and improved survival (risk reduction of 17%) irrespective of the type of bisphosphonate and osteoporosis versus cancer dose, ER positivity, node status, and presence/absence of chemotherapy.
No Advantage of Postneoadjuvant Zoledronic Acid in Primary Breast Cancer2
The NaTaN (Neo-Adjuvant Trial Add-On) study evaluated the effects of postneoadjuvant treatment with zoledronic acid in patients without pathological complete response (pCR) after antracycline-taxane- based chemotherapy for primary breast cancer. Patients were randomized within 3 months, 1 year, 2 years, or 3 years after surgery to receive intravenous (IV) zoledronic acid 4 mg with 1,000 mg calcium and 880 international unit (IU) vitamin D daily versus observation. For the first 6 months, zoledronic acid was administered every 4 weeks for the first six doses, every 3 months for the following 2 years (eight doses), and every 6 months for the last 2.5 years (five doses). Primary outcome was event- free survival (EFS); reported secondary outcomes were overall survival (OS), EFS in subgroups, and toxicity. An interim analysis was conducted with a nonprotocolled Bayesian futility analysis with a 15% futility boundary for the likelihood the results will become statistically significant. The probability of success was <6%; therefore, results were considered final and released. No EFS improvements were seen for patients given 5-year zoledronic acid postneoadjuvant therapy (HR = 0.960, 95% confidence interval [CI]: 0.709–1.30; p = .7885) in comparison to the control group. EFS benefits were not seen in any of the subgroup analyses. Similarly, no significant difference in OS (p = .4082) was noted. In addition, serious adverse events occurred more often in the treatment group (60 events) compared with the observation group (21 events). Although this first randomized postneoadjuvant zoledronic acid treatment study did not improve outcomes in patients without pCR after neoadjuvant chemotherapy, several postneoadjuvant treatment options are currently under investigation, such as rucaparib (PARP-inhibitor) in triple-negative breast cancer (BRE09-146), trastuzumab emtansine in HER2+ disease (OT1-1-06), and palbocicilib in HR+/ HER2- disease (OT2-6-11).
Aromatase Inhibitors (AIs): Breast Cancer Prevention
Anastrozole for Prevention in Postmenopausal Women at High Risk3
The International Breast Cancer Intervention Study II (IBIS-II) trial, a multicenter, randomized, placebo-controlled study, assessed the efficacy of anastrozole versus placebo in 3,864 postmenopausal women who do not have breast cancer but have an increased risk of developing breast cancer. Increased risk was determined by age, family history, type (atypia/lobular carcinoma in situ), breast density, or if the Tyrer-Cuzick model indicated a 10-year risk of breast cancer greater than 5%. The updated data with a median follow-up of 5 years were presented at the meeting. A 53% reduction in breast cancer was seen in the anastrozole treatment group (95% CI: 0.32–0.68; p < .0001). In addition, significant reductions were also seen in ductal carcinoma in situ (DCIS; 70% reduction, HR =0.30 [0.12–0.74]), all invasive breast cancer (50% reduction, HR = 0.50 [0.32–0.76]), and ER+ invasive breast cancer (58% reduction, HR = 0.42 [0.25–0.71]), but not in ER-invasive breast cancer. Compliance was similar in anastrozole and placebo groups with few dropouts due to side effects. Musculoskeletal events (63.9% versus 57.8%) and vasomotor/gynecological adverse effects (56.8% versus 49.4%) were common and significantly higher in the anastrozole group compared with placebo. Currently, the IBIS-II trial provides evidence to support the use of anastrozole for prevention in high-risk postmenopausal women. The long- term follow-up of these patients will determine the full scope of benefits and risks of anastrozole.
AI: Adverse Effects Management
Exercise Interventions to Alleviate Aromatase Inhibitors’ Arthalgia4
AIs are standard of care for hormone receptor-positive breast cancer; however, side effects, such as arthralgia, result in poor adherence and early discontinuation. The HOPE (Hormone and Physical Exercise) study examined 121 postmenopausal stage I-IIIC breast cancer patients who were taking an AI for at least 6 months and experienced at least mild arthralgia defined by pain score >3 on the Brief Pain Inventory-Short Form (BPI). The BPI measured worst pain, pain severity, and pain interference at baseline, 6, and 12 months reported on a scale of 0–10 (mild pain = 3–4, moderate pain = 5–7, severe pain = 8–10). The patients were randomized to the exercise group (n = 61) or usual care group (n = 60). The year-long exercise program consisted of a twice weekly supervised strength training session comprising six common strength-training exercises (8–12 repetitions, three sets) and 2.5 hours/week of moderate aerobic exercise with heart rate monitors to determine intensity. At baseline both controls and exercisers reported similar BPI scores with the worst pain at a pain score of 6 (moderate pain). After 12 months, women randomized to exercise had a significant (20%) decrease in their worst joint pain score, while the usual care control group had only a 3% decrease (p = .017). Women who had 80% or higher adherence to the exercise plan had greater benefits compared with women who had <80% adherence to exercise plans. This preliminary study showed clinical benefit with the use of exercise to reduce pain level from AI-induced arthralgias in breast cancer survivors; therefore, this regimen may lead to improvements in AI adherence, quality of life, and mortality risks. Additional analyses are in progress to further evaluate the benefits of this exercise intervention.
AIs: Preemptive Symptom Management May Improve Adherence5
The COBRA (Consortium on Breast Cancer Pharmacogenomics) investigators analyzed the patient-reported symptoms prior to AI initiation and after discontinuation to determine if a patient’s baseline symptoms may impact persistence with AI treatment. The ELPh (Ex- emestane and Letrozole Pharmacogenetics) trial randomized 503 postmenopausal women with early stage ER+ breast cancer to ex-emestane 25 mg PO daily (n = 248) or letrozole 2.5 mg PO daily (n= 252) for 2 years. Every 1, 3, 6, 12, and 24 months, quality of life and serum hormone concentration data were collected. Quality of life assessments evaluated depression (CESD), anxiety (HADS-A), sleep (PSQI), and symptoms (BCPT). Overall, 31.2% participants discontinued treatment due to toxicity (60 patients taking letrozole and 80 patients on exemestane). Poor sleep (45%, OR = 1.91, p = .002), feeling tired (58%, OR = 1.76, p < .001), and forgetfulness (46%, OR = 1.66, p= .015) were significant adverse effects associated with early AI treatment discontinuation. A significant correlation (p = .007) was seen between baseline symptom burden and AI discontinuation in 1 year. In conclusion, the ELPh study suggested that up-front evaluation and management of initial symptoms might help improve adherence to AI therapy by managing these symptoms before they become problematic and also identifying patients at higher risk of treatment discontinuation in order to enable appropriate interventions.
HER2+ Breast Cancer
Pathological Complete Response (pCR) Correlates with Survival Advantage in HER2+ Breast Cancer6
The initial findings of the Neo-Adjuvant Lapatinib and/or Trastu- zumab Treatment Optimisation (NeoALTTO) trial presented at SABCS in 2011 showed that the pCR rate was significantly higher in the combination lapatinib plus trastuzumab arm compared with the single therapy with either lapatinib or trastuzumab. The updated survival analyses presented at the 2013 meeting demonstrated that patients who achieved pCR had significantly better EFS and OS compared with no pCR irrespective of treatment arm. At a median follow-up of 4 years, there was a significant increase in EFS in patients who experienced pCR in comparison to no pCR (HR = 0.8 [0.22–0.63], p =.0003). In evaluating OS, a 65% reduction (HR = 0.35 [0.15–0.70], p =.005) in death risk was seen in patients who had pCR compared with patients who did not. A greater difference in EFS and OS between pCR and no pCR was seen for hormone receptor-negative breast cancer. No differences in EFS or OS were noted between the treatment arms; however, the NeoALTTO study was not powered to detect modest differences in survival. This question will be addressed by the ALTTO trial, which will be presented next year. Although the data are promising, it might be still premature to use the combination anti-HER2 therapy as a standard of care because of increased risk of adverse effects and lower adherence to therapy with the combination therapy even in hormone receptor-negative cancer. The standard of care in the neoadjuvant setting still remains chemotherapy and trastuzumab. Follow-up analysis will occur over the next 2.5 years. The results from this study also support the FDA’s accelerated approval process in the neoadjuvant setting based on the pCR data.
Neoadjuvant Lapatinib Plus Trastuzumab in Combination with Chemotherapy: Similar pCR Rates, More Toxicities, and Less Adherence Compared with Trastuzumab Only7
The TRIO-US B07 was a randomized phase 2 trial comparing the efficacy and safety of neoadjuvant lapatinib plus trastuzumab, lapatinib alone, and trastuzumab alone in HER2+ stage I–III breast cancer. Women were randomized into three treatment arms: trastuzumab (n = 34), lapatinib (n = 36), or combination lapatinib plus trastuzumab (n = 58) for 21 days followed by six cycles of Docetaxel 75 mg/m2 and carboplatin AUC 6 IV every 3 weeks. Biopsies were collected at baseline, after run-in cycle, and at surgery, with pCR rate as the primary endpoint. Although pCR was higher in lapatinib plus trastuzumab arm (52%) compared with trastuzumab (47%) or lapatinib (25%) arms as shown by other groups, the difference was only statistically significant when comparing the combination with lapatinib. Combination therapy was associated with higher incidence of diarrhea compared with trastuzumab; whereas cardiac events were not significantly different in lapatinib plus trastuzumab arm compared with trastuzumab only. Completion of therapy was significantly lower in the combination therapy (73%) and lapatinib (72%) groups compared with trastuzumab (100%). The lack of benefit from the combination therapy over the trastuzumab only arm, which has been observed in several other studies (e.g., NeoALTTO, TBCRC 006), may be due to a short therapy with anti-HER2 agents.
Novel Agents in Breast Cancer
Src Inhibitor: Dasatinib8
In a randomized study, 120 patients with ER+, HER2- breast cancer were treated with either letrozole plus dasatinib or letrozole alone as first-line therapy for metastatic disease. Time to progression was doubled from 9.7 months to 20.1 months when dasatinib was added to letrozole. This was despite no clinical benefit based on tumor size and symptoms. These findings suggest a role of dasatinib in overcoming resistance to AIs. Dasatinib might be an attractive candidate for further investigation in this setting because it also did not greatly increase the toxicity profile of the AI.
Anti-VEGF Receptor-2 Antibodies: Ramucirumab9
Ramucirumab, a recombinant human IgG1 monoclonal antibody that binds the extracellular domain of VEGF receptor-2, was investigated as a first-line therapy in combination with docetaxel for HER2- metastatic breast cancer. Progression-free survival was not significantly better in docetaxel plus ramucirumab compared with docetaxel alone (HR = 0.88; p = .08). Ongoing molecular analysis from this study may reveal biomarkers for ramucirumab response.
PARP Inhibitors: Veliparib10,11 and BMN 67312
Veliparib plus carboplatin every 3 weeks plus weekly paclitaxel therapy for a total of 12 weeks followed by AC (four cycles of doxorubicin plus cyclophosphamide [every 2–3 weeks]) regimen was tested in comparison to weekly paclitaxel followed by AC in an adaptive trial with 116 high-risk HER2- patients.10 There was a significant increase in the estimated pCR rate from 22% to 33% when veliparib plus paclitaxel were added to the neoadjuvant regimen. The increase in the estimated pCR rate was primarily observed in triple negative breast cancer (26% versus 52%) and not in hormone receptor-positive and HER2- breast cancer (14% versus 19%). Unfortunately, the relative contributions of veliparib and carboplatin could not be determined based on the study design. In a second smaller study, veliparib was tested in 20 stage IV breast cancer patients who were BRCA 1 or 2 carriers.11 At least partial response was seen in 14 of 20 patients. Another PARP inhibitor, BMN 673, was also tested in patients with deleterious germline BRCA 1 and 2 mutations.12 Partial response was achieved in 8 of 18 patients treated with BMN 673. Both veliparib and BMN 673 remain attractive candidates for further investigation in breast cancer.
References
1. Coleman R, Gnant M, Paterson A, et al. Effects of bisphosphonate treatment on recurrence and cause-specific mortality in women with early breast cancer: A meta-analysis of individual patient data from randomised trials. Presented at San Antonio Breast Cancer Symposium, Dec 10-Dec 14, 2013: Abstract [S4-07].
2. von Minckwitz G, Rezai M, Eidtmann H, et al. Postneoadjuvant treatment with zoledronate in patients with tumor residuals after anthracyclines-taxane-based chemotherapy for primary breast cancer—The phase III NATAN study (GBG 36/ABCSG XX). Presented at San Antonio Breast Cancer Symposium, Dec 10-Dec 14, 2013: Abstract [S5-05].
3. Cuzick J, Sestak I, Forbes JF, et al. Breast cancer prevention using anastrozole in postmenopausal women at high risk. Presented at San Antonio Breast Cancer Symposium, Dec 10-Dec 14, 2013: Abstract [S3-01].
4. Irwin ML, Cartmel B, Gross C, et al. Randomized trial of exercise vs. usual care on aromatase inhibitor-associated arthralgias in women with breast cancer: The hormones and physical exercise (HOPE) study. Presented at San Antonio Breast Cancer Symposium, Dec 10-Dec 14, 2013: Abstract [S3-03].
5. Henry NL, Kidwell K, Hayes DF, et al. Associations between baseline patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor (AI) therapy. Presented at San Antonio Breast Cancer Symposium, Dec 10-Dec 14, 2013: Abstract [S3-02].
6. Piccart-Gebhart M, Holmes AP, de Azambuja E, et al. The association between event-free survival and pathological complete response to neoadjuvant lapatinib, trastuzumab or their combination in HER2-positive breast cancer. Survival follow-up analysis of the NeoALTTO study (BIG 1-06). Presented at San Antonio Breast Cancer Symposium, Dec 10-Dec 14, 2013: Abstract [S1-01].
7. Hurvitz SA, Miller JM, Dichmann R, et al. Final analysis of a phase II 3-arm randomized trial of neoadjuvant trastuzumab or lapatinib or the combination of trastuzumab and lapatinib, followed by six cycles of docetaxel and carboplatin with trastuzumab and/or lapatinib in patients with HER2+ breast cancer (TRIO-US B07). Presented at San Antonio Breast Cancer Symposium, Dec 10-Dec 14, 2013: Abstract [S1-02].
8. Paul D, Vukelja SJ, Holmes FA, et al. Letrozole plus dasatinib improves progression-free survival (PFS) in hormone receptor- positive, HER2-negative postmenopausal metastatic breast cancer (MBC) patients receiving first-line aromatase inhibitor (AI) therapy. Presented at San Antonio Breast Cancer Symposium, Dec 10-Dec 14, 2013: Abstract [S3-07].
9. Mackey JR, Ramos-Vazquez M, Lipatov O, et al. Primary results of ROSE/ TRIO-12, a randomized placebo controlled phase III trial evaluating the addition of ramucirumab to first-line docetaxel chemotherapy in metastatic breast cancer. Presented at San Antonio Breast Cancer Symposium, Dec 10-Dec 14, 2013: Abstract [S5-04].
10. Rugo HS, Olopade O, DeMichele A, et al. Veliparib/carboplatin plus standard neoadjuvant therapy for high-risk breast cancer: First efficacy results from the I-SPY 2 TRIAL. Presented at San Antonio Breast Cancer Symposium, Dec 10-Dec 14, 2013: Abstract [S5-02].
11. Somlo G, Frankel P, Luu T, et al. Efficacy of ABT-888 (veliparib) in patients with BRCA-associated breast cancer. Presented at San Antonio Breast Cancer Symposium, Dec 10-Dec 14, 2013: Abstract [P2-16-05].
12. Mina LA, Ramanathan RK, Wainberg ZA, et al. BMN 673 is a PARP inhibitor in clinical development for the treatment of breast cancer patients with deleterious germline BRCA 1 and 2 mutations. Presented at San Antonio Breast Cancer Symposium, Dec 10-Dec 14, 2013: Abstract [P2-09-02].
The Resident’s Cubicle
Alex Shillingburg, PharmD
Clinical Specialist BMT/ Hematologic Malignancy
WVU Healthcare, Morgantown, WV
Surviving the Interview Season
It’s open season for potential residency candidates and new job seekers. This edition of The Resident’s Cubicle will focus on tips to help PGY2 oncology residents survive the impending interview season. There are three facets of the interview season that have a significant impact on the oncology resident’s life: traveling to and preparing for job interviews, keeping up with residency responsibilities while absent from work for interviews and travel, and interviewing oncology residency candidates to take your place in your program the following year.
Preparing for the Job Interview
The time has come to find a place to work for an indefinite period of time. Many things need to play into this decision, such as location, family circumstances, fit with the mission and atmosphere of the institution, and the job responsibilities. Here are some tips that are important enough to reiterate even though they should be second nature after all the interviewing over the past few years to get to this point.
- Talk. It may seem silly, but to figure out if you would be a good fit, you need to be engaged during your interview. If you don’t engage, not only will you seem dull, but you won’t be able to get a good sense of the organization. Talk to the cab driver, the cashier in the lunch line, and the receptionist. Everyone you meet can help mold your opinion of a place. Don’t talk over your interviewers, but be sure to take advantage of every chance to interact. You are only there for a short period and want to gather as much information as you can to make an informed decision.
- Be real. Be yourself and be honest. It should go without saying to absolutely not falsify any information and to be honest about your personality. Don’t be the person you think they want; be who you are. You will thank yourself later when the real you fits well in your new position!
- Dress smart. Put your absolute best foot forward for your first impression. There is no need to be a fashionista, but always wear a matching freshly pressed suit; wear clean, unscuffed shoes; and keep hair, facial hair, nails, and accessories well-groomed, neat, and tasteful.
- Never be late. Plan for weather-related delays, especially in the winter.
- Be interested. Ask plenty of questions to which you genuinely want answers. Nothing turns someone off more than interviewing a candidate who doesn’t want to be there. Nonverbal cues send very strong signals. Sit up straight, lean in slightly, make eye contact, and smile.
During your interview you will make connections with professionals in your future field and, trust me, you will see them again. Oncology pharmacy is a small world, and it never hurts to have friends in many institutions.
One aspect of job interviewing that will differ drastically from residency interviewing will be the inconsistent timeline. This is often the most frustrating part of the job search for PGY2 residents. There will not be a magical match day when you find out with which institution you were matched. There will not be a tight 6-week timeline for interviews. You will likely find that some places may take weeks to months to evaluate and interview all of their candidates. Job offers could be made to you before you even go to other interviews, and they may expect an answer quickly. Navigating this very unpredictable timeline will require difficult life decisions, often without all of the pieces of the puzzle in front of you. My best advice to you is to be professional. Be upfront with your intentions, respect the institution’s time frame as well as your own, and don’t accept a job expecting to reject it if a better one comes along. If your top institution has not made an offer yet and you need to give other places an answer, it is not unreasonable to contact human resources to ask if they have filled the position or know when they will begin making offers. Deciding whether to take a good offer or to turn it down to gamble on an offer from your dream job is difficult. Unfortunately there is no right answer, but be prepared for these situations to arise and take care not to burn bridges.
Keeping Up During Your Absence
Don’t assume that your responsibilities to your residency will be absolved when you are away for an interview. Regardless of the number of days missed for interviews, residents should expect to be held accountable for their residency expectations during this time. That’s not to say that you should cancel interviews or that special accommodations cannot be made for extra days away from the residency. The best way to address missed days is to keep open and upfront communication with your residency program director and your preceptors during this time. It is likely that they will know how many positions you have applied for because you may be asking them for recommendations, but be forthcoming with interview dates and travel plans as soon as possible. Adjustments may need to be made to make up presentations or activities after a rotation has ended. Topic discussions may be concentrated during specific times during rotations to maximize learning with limited days on rotation. Additional activities may be necessary if significant patient care time is missed, but the resident’s learning experience should not be impacted by excessive absences.
Keep in mind when scheduling interviews that middle-of-the-week dates will require more time off for travel but may not be avoidable. Be sure to maintain an up-to-date agenda during this time, and always inform the organizer of any meetings or presentations you will miss. Try to reschedule to keep your original commitments and work with colleagues to help cover any duties you have scheduled such as precepting students, teaching classes, or maintaining operational responsibilities. These things can often be easily addressed in advance, but will reflect extremely poorly on you if forgotten.
Managing PGY2 Candidate Interviews at Your Program
Regardless of your role in these interviews—dinner host, tour guide, or planned one-on-one interview—do not discount their importance. Even though you may be leaving in a few short months, do not forget that the candidate who matches will graduate from the same program you did. You should have pride in your program and work to ensure that the candidate who will replace you is the best fit for your program.
These interviews can also take a significant amount of time from your day depending on your interview responsibilities, which you should consider while managing your to-do list for that day. It can also be very exhausting to be consistently “on” if you are with the candidate for long periods of time throughout the day. It is crucial to be as engaged during the last few minutes while walking them out as you were in the morning when they first stepped foot in the building. Your excitement and professionalism will leave an impression with the candidate, and you want that impression to be that you are prompt, cordial, respectful of them, and proud to be graduating from your program.
Be prepared with three or four questions to ask each candidate so that you have an equal basis on which to compare. Remember to take notes of their responses, questions they asked you, and your impression. You will be surprised by how many faces you will see during these months, and you will need your notes to jog your memory come rank time. Don’t be surprised if you don’t get to all of your questions because the candidate may be prepared with a continuous stream of his or her own questions for you. Almost everyone will advise them to talk to the current resident. You are in the midst of the program they are interested in and the most relatable source of information. Be sure to be truthful but keep in mind that this is not the time to vent your frustrations. You represent your institution and your pro- gram. It is appropriate to talk about recommendations for improvements, but complaining or trash-talking will only reflect poorly on you.
In summary, this time will be very busy and will require oncology residents to continually switch gears and refocus from interviewer to interviewee. Always be mindful of the situation you are in and maintain a professional attitude. Through all the stress and commotion, this is also an extremely exciting time with big changes and opportunities on the horizon. Explore every option, keep an open mind, and stay excited about the novelty of new people, cities, and jobs. You have worked hard to get to this point. Be proud of your accomplishments!
